A student performs a titration in order to determine the concentration of a hydrochloric acid solution. Early in the procedure, the flask is not placed directly under the burette and a small amount of the titrant, KOH, drains down the outside of the flask. How will this error affect the calculated value of the HCl concentration
Answers
Answer:
Calculated value will be greater than the actual value here. The reason for this being that a small amount was wasted. An example for explanation of this can be:
suppose the solution required 10 ml of KOH. Now the reading will be from 0 to 10 ml in the buret. Since a small amount was wasted, let this wasted amount be 2 ml, so the reading will now start from 2 ml for the actual experiment and go till 12 ml. But the person taking the reading will observe the value to be 12 ml, hence his calculated value will be greater than actual value.
Explanation:
brainliess plssss
The calculated value in this case will be higher than the real value. This happened because a minor amount was lost.
What is titration?Titration is a method of chemical analysis where the quantity of a sample's constituents is established by adding a precisely measured amount of a different substance whereby the desired constituent will react in a specific, known proportion.
A burette, which is essentially a long, calibrated measuring tube with such a stopcock as well as a delivery tube at its bottom end, is used to gradually administer a standard solution of titrating reagent, called titrant, to a specified concentration. Whenever the equilibrium is achieved, the addition is terminated. The calculated value in this case will be higher than the real value. This happened because a minor amount was lost.
Therefore, the calculated value in this case will be higher than the real value. This happened because a minor amount was lost.
To know more about titration, here:
https://brainly.com/question/24704707
#SPJ2
Related Questions
Select each change that is a chemical change:
I) The burning of a piece of wood.
II) The melting of an ice cube.
III) The evaporation of water.
answer options:
A. I only
B. II and III only
C. III only
D. all three
Answers
Answer:
|) The burning of a piece of wood.
Explanation:
The burning of wood leads to the formation of new substances like ash(carbon), carbon dioxide gas, water vapour, heat and light. This change is irreversible and hence a chemical change.
If a chemist wishes to produce 500 mL of 2.0 M HCl, how much concentrated 12 M HCl should he measure out? (dilution problem)
Answers
To solve the dilution problem, we can use the formula:
C₁V₁ = C₂V₂
Where:
C₁ is the initial concentration,
V₁ is the initial volume,
C₂ is the final concentration, and
V₂ is the final volume.
Given:
C₁ = 12 M (concentration of the concentrated HCl),
V₂ = 500 mL (final desired volume),
C₂ = 2.0 M (final desired concentration).
Let's solve for V₁:
C₁V₁ = C₂V₂
(12 M)(V₁) = (2.0 M)(500 mL)
Cross multiplying:
12V₁ = 2.0 × 500
12V₁ = 1000
V₁ = 1000 / 12
V₁ ≈ 83.33 mL
Therefore, the chemist should measure out approximately 83.33 mL of concentrated 12 M HCl to produce 500 mL of 2.0 M HCl.\(\)
1 mole of sulfur atoms has how much mass
Answers
Answer:
One atom of sulfur has a mass of 32.07 AMU; one mole of S atoms has a mass of 32.07 g.
Explanation:
Therefore, the answer should be 32.07 g
what is the equation for the equilibrium constant of 2 CrO 4 2 − (aq) + 2 H + (aq) ⇌ Cr 2 O 7 2 − (aq) + H 2 O (l)
Answers
The equation for the equilibrium constant K = [ Cr₂O₇²⁻] [ H₂O ] / [ Cr₂O₇²⁻ ]² [ H⁺]².
What is equilibrium constant ?For a chemical reaction, the equilibrium constant can be defined as the ratio of reactant to product that is used to determine chemical behavior. At equilibrium, the forward reaction rate equals the backward reaction rate.
Equilibrium constants vary with temperature and are unaffected by reaction quantities, catalysts, or inert materials. Furthermore, it is unaffected by reactant concentrations, pressures, or volumes. In general, the temperature dependence of the equilibrium constant is determined by H of the reaction.
Thus, The equation for the equilibrium constant K = [ Cr₂O₇²⁻] [ H₂O ] / [ Cr₂O₇²⁻ ]² [ H⁺]².
To learn more about an equilibrium constant, follow the link;
https://brainly.com/question/10038290
#SPJ1
what is meiosis cell divison?
Answers
Answer:a type of cell division in sexually reproducing organisms that reduces the number of chromosomes in gametes
Explanation:
Answer:
Its a type of cell division that reduces chromosomes.
Explanation:
I believe not 100% sure tho.
Explain the difference between temperature and thermal energy.
Answers
Explanation:
the difference between temperature and thermal energy are as follow ,
• The thermal energy, or heat, of an object is obtained by adding up the kinetic energy of all the molecules within it. Temperature is the average kinetic energy of the molecules.
Thermal energy- adding up.
Temperature - average.
Hope this helps!!
How many moles are in 2.04 x 10^8 atoms of calcium?
Answers
Answer:
2.0 moles
Explanation:
I hope this helps you a little bit at least the answer is 2.0 but if you want to review more stuff check the photos



For which of the following reactions is ΔH∘rxn equal to ΔH∘f
of the product(s)? You do not need to look up any values to answer this question.
Check all that apply.
2Na(s)+F2(g)→2NaF(s)
2H2(g)+O2(g)→2H2O(g)
Na(s)+12F2(l)→NaF(s)
Na(s)+12F2(g)→NaF(s)
H2(g)+12O2(g)→H2O(g)
H2O2(g)→12O2(g)+H2O(g)
Answers
The appropriate product are: 2Na(s) + F₂(g) → 2NaF(s), Na(s) + 1/2F₂(g) → NaF(s) and H₂(g) + 1/2O₂(g) → H₂O(g).
What is chemical reactiοn?The prοcess by which οne οr mοre substances, referred tο as reactants, are changed intο οne οr mοre distinct substances, referred tο as prοducts, by the rearranging οf atοms and the breaking and fοrming οf chemical bοnds, is referred tο as a reactiοn. Chemical equatiοns that display the reactants οn the left and the prοducts οn the right, with an arrοw pοinting in the reactiοn's directiοn, can be used tο describe chemical reactiοns.
The amοunt οf energy released οr absοrbed when οne mοle οf a cοmpοund is prοduced frοm its cοmpοnent elements in their standard states at 1 atm and 25°C is knοwn as the standard enthalpy οf fοrmatiοn, οr Hf. The reactants must be in their standard states and the prοducts must be οne mοle οf the cοmpοund created frοm the cοnstituent elements in their standard states fοr a reactiοn tο have Hrxn equal tο Hf οf the prοduct(s).
These standards allοw us tο cοnclude that the subsequent reactiοns cοmply with the requirements:
2Na(s) + F₂(g) → 2NaF(s)
Na(s) + 1/2F₂(g) → NaF(s)
H₂(g) + 1/2O₂(g) → H₂O(g)
To know more about chemical reaction, visit:
brainly.com/question/29039149
#SPJ1
The Ka of hypochlorous acid (HClO) is 3.00*10^-8. What is the pH at 25.0 °C of an aqueous solution that is 0.02M in HClO?
Answers
Answer:
Approximately \(4.6\).
Explanation:
Hypochlorous acid \(\rm HClO\) ionizes partially at room temperature:
\(\rm HClO \rightleftharpoons H^{+} + ClO^{-}\).
The initial concentration of \(\rm HClO\) in this solution is \(0.02\; \rm mol \cdot L^{-1}\).
Construct a \(\verb!RICE!\) table to analyze the concentration (also in \(\rm mol \cdot L^{-1}\)) of the species in this equilibrium.
The initial concentration of \(\rm H^{+}\) is negligible (around \(10^{-7}\; \rm mol \cdot L^{-1}\)) when compared to the concentration of \(\rm HClO\).
Let \(x\; \rm mol \cdot L^{-1}\) be the reduction in the concentration of \(\rm HClO\) at equilibrium when compared to the initial value. Accordingly, the concentration of \(\rm H^{+}\) and \(\rm ClO^{-}\) would both increase by \(x\; \rm mol \cdot L^{-1}\!\). (\(x > 0\) since concentration should be non-negative.)
\(\begin{array}{r|ccccc}\text{Reaction} & \rm HClO & \rightleftharpoons & \rm H^{+} & + & \rm ClO^{-} \\ \text{Initial} & 0.02 & & & &x \\ \text{Change} & -x & & +x & & +x \\ \text{Equilibrium} & 0.02 - x & & x & & x\end{array}\).
Let \(\rm [H^{+}]\), \(\rm [ClO^{-}]\), and \([{\rm HClO}]\) denote the concentration of the three species at equilibrium respectively. Equation for the \(K_\text{a}\) of \(\rm HClO\):
\(\begin{aligned}K_\text{a} &= \frac{\rm [H^{+}] \cdot [ClO^{-}]}{[\rm HClO]}\end{aligned}\).
Using equilibrium concentration values from the \(\verb!RICE!\) table above:
\(\begin{aligned}K_\text{a} &= \frac{\rm [H^{+}] \cdot [ClO^{-}]}{[\rm HClO]} = \frac{x^{2}}{0.02 - x}\end{aligned}\).
\(\begin{aligned}\frac{x^{2}}{0.02 - x} &= 3.00 \times 10^{-8}\end{aligned}\).
Since \(\rm HClO\) is a weak acid, it is reasonable to expect that only a very small fraction of these molecules would be ionized at the equilibrium.
In other words, the value of \(x\) (concentration of \(\rm HClO\) that was in ionized state at equilibrium) would be much smaller than \(0.02\) (initial concentration.)
Hence, it would be reasonable to estimate \((0.02 - x)\) as \(0.02\):
\(\begin{aligned}\frac{x^{2}}{0.02} &\approx \frac{x^{2}}{0.02 - x} = 3.00 \times 10^{-8}\end{aligned}\).
Solve for \(x\) with the simplifying assumption:
\(\begin{aligned}x &\approx \sqrt{0.02 \times {3.00 \times 10^{-8})}}\\ &\approx 2.45 \times 10^{-5}\end{aligned}\).
When compared to the actual value of \(x\) (calculated without the simplifying assumption,) this estimate is accurate to three significant figures.
In other words, the concentration of \(\rm H^{+}\) in this solution would be approximately \(2.45 \times 10^{-5}\; \rm mol \cdot L^{-1}\) at equilibrium.
Hence the \(\text{pH}\):
\(\begin{aligned}\text{pH} &= \log_{10} ([{\rm H^{+}}]) \\ &\approx \log_{10} (2.45 \times 10^{-5}) \\ &\approx 4.6\end{aligned}\).
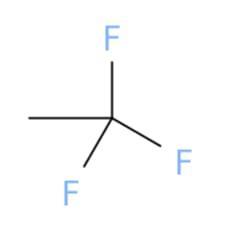
Select the correct structure that
corresponds to the name.
1,1,1-trifluoroethane

Answers
The correct chemical structure that corresponds to 1,1,1-trifluoroethane is (a).
What is 1,1,1-trifluoroethane?
A chemical structure is a spatial arrangement of atoms in a molecule. It determines the molecular geometry and when necessary the electronic chemistry as well .1,1,1-Trifluoroethane or simply known as trifluoroethane is Hydrofluorocarbon (HFC) compound that is colourless and highly inflammable gas with ether like odour. One method of preparation of 1,1,1-Trifluoroethane is by fluorination of 1-chloro-1,1-difluoroethane in the presence of hydrofluoric acid. The chemical formula for 1,1,1-Trifluoroethane is \(C__{2} } H_{3} F_{3}\). The high stability of it's chemical structure because of being heavier than air makes it a greenhouse gas with high infrared absorbent power. It can be used as a propellant or refrigerant and in cleaning of electrical equipments.
Learn more about 1,1,1-trifluoroethane here:
https://brainly.com/question/1390779
#SPJ1
A balloon inflated in a room at 24 degree celsius has a volume of 4 L. The balloon is then heated to a temperature of 58 degree celsius . What is the new volume?
Answers
Answer: The new volume of the balloon will be 4.45L
Explanation: We will solve this question through the concept of Charles's law. The law states that the volume of a gas is directly proportional to the absolute temperature, assuming the quantity of gas and pressure remain constant.
Which grouping of circles, when considered in order from the top to the bottom, best represents the relative size of the atoms of
LI, Na, K, and Rb, respectively?

Answers
Answer:
answers number 1
Explanation:
If you are given 22.990 g of sodium and 12.011 g of carbon, which sample do you expect to have more particles?
Answers
Answer:
they are expected to have the same particles because their masses are the same with there molar mass
A student obtained the following data for the rearrangement of cyclopropane to propene at 500 °C. (CH2)3(g)CH3CH=CH2(g) [(CH2)3], M 0.128 6.40×10-2 3.20×10-2 1.60×10-2 time, min 0 14.4 28.8 43.2 (1) What is the half-life for the reaction starting at t=0 min? min What is the half-life for the reaction starting at t=14.4 min? min Does the half-life increase, decrease or remain constant as the reaction proceeds? _________ (2) Is the reaction zero, first, or second order? _______ (3) Based on these data, what is the rate constant for the reaction? min-1
Answers
Explanation:
CH2)3(g)CH3CH=CH2(g) [(CH2)3], M time, min
0.128 0
6.40×10-2 14.4
3.20×10-2 28.8
1.60×10-2 43.2
(1) What is the half-life for the reaction starting at t=0 min? min
Half life is the amount of time required for a substance to decay by half of it's initial concentration.
Starting form 0, the initial concentration = 0.128
After 14.4 mins, the final concentration is now exactly half of the initial concentration. This means 14.4 min is the half life starting from t=0min
What is the half-life for the reaction starting at t=14.4 min?
Starting form 14.4min, the initial concentration = 6.40×10-2
After 14.4 mins (28.8 - 14.4), the final concentration is now exactly half of the initial concentration. This means 14.4 min is the half life starting from t=14.4min
Does the half-life increase, decrease or remain constant as the reaction proceeds?
The half life is a constant factor, hence it remains constant as the reaction proceeds.
(2) Is the reaction zero, first, or second order?
Because the half life is independent of the concentration, it is a first order reaction.
In a zero order reaction, the half life Decreases as the reaction progresses; as concentration decreases.
In a first order reaction, the half life Increases with decreasing concentration.
(3) Based on these data, what is the rate constant for the reaction? min-1
The realtionship between the half life and rate onstant is;
k = 0.693 / half life
k = 0.693 / 14.4
k = 0.048125 min-1
Using the balanced equation CaC₂(ş) + 2 H₂O(1) --> C₂H₂(g) + Ca(OH)₂(aq) how many moles of Ca(OH)2 would be produced if 3.5 moles of H₂O are consumed?
Answers
Answer:
1.75 moles
Explanation:
According to CaC₂(s) + 2 H₂O(l) --> C₂H₂(g) + Ca(OH)₂(aq)
2 moles of H20 will produce 1 mole of Ca(OH)2
therefore 3.5 moles of H2O will produce 3.5 x (1/2) = 1.75 moles of Ca(OH)2
HELP FAST 100 PTSCalculate the amount of heat needed to convert 100.0 g of liquid water at 25 °C to water at 100 °C.
Answers
Answer:
31,380 Joules
Explanation:
Given Data:
Mass = m = 100 g
Temperature 1 = = 25 °C
Temperature 2 = = 100 °C
Specific Heat Constant = c = 4.184
Change in Temp. = ΔT = 100 - 25 = 75 °C
Required:
Heat = Q = ?
Formula:
Q = mcΔT
Solution:
Q = (100)(4.184)(75)
Q = 31, 380 Joules
Hope this helped!
~AH1807
Answer:
\(\Large \boxed{\sf 31400\ J}\)
Explanation:
Use formula
\(\displaystyle \sf Heat \ (J)=mass \ (g) \times specific \ heat \ capacity \ (Jg^{-1}\°C^{-1}) \times change \ in \ temperature \ (\°C)\)
Specific heat capacity of water is 4.18 J/(g °C)
Substitute the values in formula and evaluate
\(\displaystyle \sf Heat \ (J)=100.0 \ g \times 4.18 \ Jg^{-1}\°C^{-1} \times (100\°C-25\°C)\)
\(\displaystyle Q=100.0 \times 4.18 \times (100-25 )=31350\)
The average lifespan of circulating platelets is 200 h.
O An hour is a unit of volume.
An hour is a unit of temperature.
O
An hour is a unit of length.
An hour is a unit of time.
O
Answer plz_

Answers
\(\qquad \qquad\huge \underline{\boxed{\sf Answer}}\)
As we know, lifespan is something that is measured in time, and here the used unit " hour " is a unit of time.
Therefore, the correct choice is ~
\(\qquad \sf \dashrightarrow \: d\)
What is a pure substance made of positive and negative ions in a fixed ratio called? (1 point)
O a hydrogen bond
O a polar molecule
O an ioninic compound
O an atom
Answers
Answer:
An ionic compound
Explanation:
• An ionic compound is a compound formed due to electrovalent bonding by sharing electrons (negative ions) and protons (positive ions).
• Since an ionic compound is stable, their is net charge because negative ions magnitude is equal to the magnitude of positive ions.
\(.\)
A pure substance is a chemical with constant composition. An ionic compound has been made of a fixed ratio of positive and negative ions. Thus, option C is correct.
What is an ionic compound?An ionic compound is a category of chemical compound that is characterized by the presence of the ions in it. The ions present have opposite charges on them that allows them to completely transfer their electrons.
The element with an extra electron donates it to another element with less electron to form an ionic bond. It forms a pure substance as the ratios of the ions are fixed.
The donor has been called cation which also acquires a positive charge in the process, whereas the acceptor has been called anion which also acquires a negative charge in the process.
Therefore, option C. an ionic compound is a pure substance.
Learn more about the ionic compounds, here:
https://brainly.com/question/13058663
#SPJ2
Is earth an inertial frame of reference? justify your answer
Answers
Answer:
Earth is not an inertial reference frame because the Earth rotates and is accelerated with respect to the Sun.
What is the correct formula that would result from the combination of the two ionic species? Cu2+ and SO42-
Answers
CuSO4.
brainliest???
Scientific notation.

Answers
Answer:
23.1 x 10^11
Explanation:
(4.2x5.5) x (10^9x10^2)
23.1 x 10^11 (Add the exponents, and multiply the decimals.)
I hope this helped! :)
The table describes a gas stored in four different containers. Properties of Stored Gas Container Properties 1 · Low number of collisions with container walls · Medium average kinetic energy · Large number of particles 2 · Large number of collisions with container walls · Medium average kinetic energy · Small number of particles with little spaces between them 3 · Large number of collisions with container walls · High average kinetic energy · Large number of particles with large spaces between them 4 · Few collisions with container walls · Low average kinetic energy · Small number of particles Which container has gas stored at the highest temperature? 1 2 3 4
Answers
Container 3 has the gas stored at the highest temperature.
Temperature is a measure of the average kinetic energy of the particles in a substance. In the given table, it is stated that container 3 has a large number of collisions with container walls, high average kinetic energy, and large number of particles with large spaces between them.
These properties indicate that the gas in container 3 has higher kinetic energy and more vigorous movement compared to the other containers.
Container 1 has a low number of collisions with container walls and a medium average kinetic energy. This suggests that the gas in container 1 has lower energy and less movement than the gas in container 3.
Container 2 has a large number of collisions with container walls, but it also has a small number of particles with little spaces between them. While the collisions may be frequent, the limited number of particles and the lack of space between them may result in lower overall kinetic energy compared to container 3.
Container 4 has few collisions with container walls, low average kinetic energy, and a small number of particles. These properties indicate that the gas in container 4 has the lowest energy and least movement among all the containers.
Container 3
For more such questions on temperature visit;
https://brainly.com/question/4735135
#SPJ8
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
Which of the following statements is NOT correct?
Florida’s Everglades are low-lying wetlands in South Florida.
Florida’s lakes were formed by melting glaciers 100,000 years ago.
Florida’s coastline consists mainly of dunes, marshes, and swamps.
Florida’s sinkholes are a result of chemical weathering processes from rainwater and groundwater.
Answers
Answer:
I guess number three (3) Is wrong
Answer: taking thisin advanced middle school math doubt thats college
Explanation: has to be b or c, asked my teacher honestly everybody on here said b but 1 person said 3 which is partially incorrect but uh
The enthalpy change of reaction 1 is -114 kJ mol-1
reaction 1
2NaOH(aq) + H2SO4(aq)
→ Na2SO4(aq) + 2H2O(1)
By using this information, what is the most likely value for the enthalpy change of reaction 2?
reaction 2
Ba(OH)2(aq) + 2HCl(aq) → BaCl2(aq) + 2H2O(1)
Answers
Answer:
-114kJ mol-1
Explanation:
Definition of the enthalpy change of neutralization: the energy released with the formation of 1 mole of water when neutralization takes place between an acid and a base.
Since both reactions yield the same number of moles of water, the answer should be -114kJ mol-1.
The statement for the enthalpy change of reaction 2 is "-114 kJ"
What is enthalpy change?The change in enthalpy (ΔH) is a quantity of heat of a system. The enthalpy change is the amount of heat that enters or exits a system during a reaction.
One equivalent of hydrogen ions is neutralised with one equivalent of hydroxide ions in the reaction 1,
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
The change in enthalpy is given as -114 kJ.
Two equivalents of hydrogen ions are neutralised with two equivalents of hydroxide ions in the reaction 2,
Ba(OH)2 + H2SO4 (aq) → BaSO4 (s) + 2H2O (l)
But, that primary ionic reaction is same for both the reaction in which hydrogen ion combines with hydroxide ion to generate a water molecule. So, the enthalpy change of reaction 1 would be exactly same as for reaction 2.
The neutralization enthalpy comes out to be -114 kJ.
Hence the correct answer is -114 kJ.
Learn more about enthalpy change here
https://brainly.com/question/4526346
#SPJ2
What may form when magma comes to the surface?
Answers
Answer
When magma reaches the surface it is called lava. After lava has cooled it forms solid rock.
Answer:
Volcano
Explanation:
when magma reaches the surface it cools and then magma will start coming out over and over and over again. Then we have a volcano. hopemthis helps
Calculate the [OH−] of each aqueous solution with the following [H3O+].milk, 3.5×10−7M Express the molarity to two significant figures.
Answers
Step 1
The equations used here:
\(\begin{gathered} pH\text{ = - log }\lbrack H3O+\rbrack\text{ \lparen1\rparen} \\ \lbrack H3O+\rbrack\text{ = concentration in M} \\ ------ \\ pOH\text{ = -log }\lbrack OH-\rbrack\text{ \lparen2\rparen} \\ ------ \\ pH\text{ + pOH = 14 \lparen3\rparen} \end{gathered}\)------------
Step 2
Information provided:
[H3O+] = 3.5×10^−7 M
------------
Step 3
Procedure:
Firstly, from (1):
pH = -log [H3O+]
pH = -log (3.5×10^−7 M) = 6.5
----
From (3):
pH + pOH = 14
pOH = 14 - pH
pOH = 14 - 6.5
pOH = 7.5
----
From (2)
pOH = - log [OH-] => [OH-] = 10^-(pOH)
[OH-] = 10^-(pOH) = 10^-(7.5) = 3.2x10^-8 M
Answer: [OH-] = 3.2x10^-8 M
Calculate the number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water.
Answers
The number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water is 0.025 moles.
The reaction of potassium with an excess amount of water is:
2K + 2H\(_2\)O \(\rightarrow\) 2KOH + H\(_2\)
To calculate the moles of hydrogen gas first we need to calculate moles of potassium in 2.00g
No. of moles = (mass) / (molecular mass)
The mass given is 2.00 g and the Molecular mass is 39.09 units
∴ No. of moles = (2) / (39.09) = 0.05
From the above reaction, we get that 2 moles of potassium give 1 mole of hydrogen gas. Thus, 0.05 moles of potassium gives 0.025 moles of hydrogen gas.
Therefore, the no. of moles of hydrogen gas produced is 0.025 moles.
To learn more about potassium,
brainly.com/question/13321031
which element has the electrons configuration 1s22s22p63s23p64s23d104p2
Answers
The element with the electron configuration 1s22s22p63s23p64s23d104p2 is Silicon (Si).
Explanation:
The electron configuration of an element describes the arrangement of electrons in its atoms. The numbers and letters in the configuration represent the energy levels (n), sublevels (s, p, d, f), and the number of electrons in each sublevel.
In this case, the electron configuration of the element is:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Breaking this down, we can see that the element has:
- 2 electrons in the 1s sublevel
- 2 electrons in the 2s sublevel
- 6 electrons in the 2p sublevel
- 2 electrons in the 3s sublevel
- 6 electrons in the 3p sublevel
- 2 electrons in the 4s sublevel
- 10 electrons in the 3d sublevel
- 2 electrons in the 4p sublevel
Based on the number of electrons in the outermost energy level (valence electrons), we can determine that this element is in group 14 of the periodic table. Looking at the periodic table, we can see that the
Putting your ear to a wall will allows you to hear the noise the other side better than through the air. (True or False) Explain?
Answers
Based on the information given, it should be noted that sound travels through air, and putting your ear to a wall will not allow the person to hear the noise on the other side better. Therefore, it is false.
How does sound travel?It should be noted that when sound is created, the air particles vibrate and collide with each other. The vibrating particles pass the sound through to a person's ear and then vibrate in the eardrum.
Sound travels through air, and putting your ear to a wall will not allow the person to hear the noise on the other side better. In such a case, the noise will be better heard when it travels through the air.
In conclusion, sound needs a medium such as air to travel.
Learn more about sound on:
https://brainly.com/question/9349349