Answers
Answer:
0.0451 moles ≅ 0.04 moles
Explanation:
We can calculate how many moles of calcium chloride (CaCl₂) are in 5.00 g sample, by dividing the mass into the molar mass (MM) of the compound:
moles = mass/MM
First, we calculate MM of CaCl₂ from the chemical formula, using the molar mass of the elements Ca and Cl:
MM CaCl₂ = (1 x MMCa) + (2 x MMCl) = 40 g/mol + ( 2 x 35.453 g/mol)
= 110.9 g/mol
Finally, we calculate the moles from the mass and MM:
moles of CaCl₂ = mass/(MM CaCl₂) = 5.00 g/(110.9 g/mol) = 0.0451 moles ≅ 0.04 moles
Related Questions
please Help me !! NO LINKS! fill this chemical reaction please
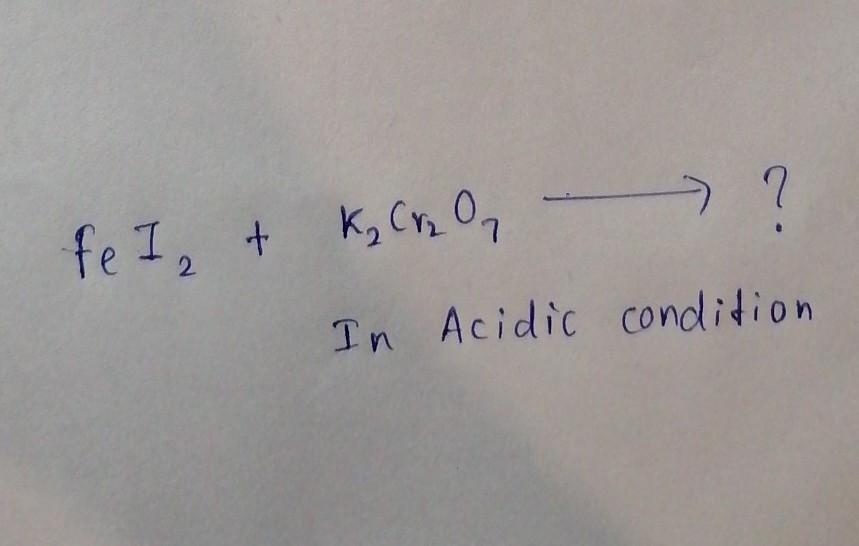
Answers
Answer:
see the above attachment.
Explanation:
hope this helps you.

yes or no ? experimentation with corn and other crops led to the development of new fuels called biofuels
Answers
Yes, the experimentation with corn and other crops led to the development of new fuels called biofuels.
What are biofuels?Biofuel is a biodegradable, inexhaustible, fuel that is produced from Biomass. Biofuel is considered the easiest available and pure fuel on the earth. Biofuels are manufactured from biomass such as wood and straw, which are liberated by direct combustion of dry matter and converted into a gaseous and liquid fuel.
The organic matter such as sludge, sewage, and vegetable oils, can be changed into biofuels by a wet process such as fermentation and digestion. Biofuel is available in all regions of the world, and mainly includes fuels such as Biodiesel, Bioethanol, and Bio methanol.
The two types of biofuels are bioethanol and biodiesel most commonly used these days. Both of these biofuels are the first generation of biofuel technology.
Learn more about biofuels, here:
https://brainly.com/question/502679
#SPJ1
a 82.6 L sample of gas exerts 350.8 mm hg pressure at 134.6 c°. what valine does the gas have at 736.4 mm hg and 42.8 c°
a. 30.5
b 4.13
c. 12.5
d. 134
need help ASAP
Answers
Answer:
nfururhrj waltz quiz amora7ersgdsYsdi6 whiz 53
96rduttie
4. Describe how you will measure the volume of a piece of stone
Answers
Answer:
One way to measure the volume of any irregular object (in your case, a stone) is to submerge it completely under water and measure the change in the height of the water level. This change in the water level (let's say it goes from 50 mL to 65 mL) indicates that the stone has a volume of 15 mL.
The compound butanol has the following structural formula.
A string of 4 C atoms are bonded above, left, and below to H. The right-hand end is bonded to O, which in turn is bonded to H.
Which of these is a structural isomer of butanol?
A string of 4 C atoms are bonded above, below, left and right to H.
A string of 4 C atoms is bonded above, below, left, and right to H, except the second C, which is bonded below to O, which is bonded below to H.
A string of 4 C atoms is bonded above, below, left, and right to H, but the chain is interrupted between the first and second C, which are bonded to an O between them.
A string of 4 C atoms is bonded above, below, and left to H, except the last C has no H below and is double-bonded to an O to the right.
Answers
The structural formula of butanol is C4H9OH. It consists of a chain of four carbon atoms, with a hydroxyl (-OH) group attached to one of the carbon atoms. Butanol has several structural isomers, which have the same molecular formula but different structural formulas.
A structural isomer is a compound that has the same molecular formula as another compound but has a different arrangement of its atoms. A string of 4 C atoms are bonded above, below, left, and right to H, except the second C, which is bonded below to O, which is bonded below to H is a structural isomer of butanol.
This is called butan-2-ol. The structural formula of butan-2-ol is CH3CH(OH)CH2CH3. In this isomer, the hydroxyl group is attached to the second carbon atom in the chain, whereas in butanol, the hydroxyl group is attached to the first carbon atom in the chain.
For more question on atoms
https://brainly.com/question/6258301
#SPJ8
1.00 mole of O2 contains the same number of oxygen atoms as?
A) 0.667 mole of O3.
B) 1.00 mole of CH3CO2H.
C) 2.00 mole of CH3CH2OH.
D) All of the above
Answers
Answer:
1.00mole of CH3CO2H
Explanation:
1mole of O2mole =26.022*10^23 atoms
gravity sample work connection’s academy
PLEASE HELP AS SOON AS POSSIBLE WILL MARK BRAINIEST
Answers
Answer:
search on YT Khan academy work done by gravity
Explanation:
you will get it for sure with best explanation
mark me please on brainlest
Equal masses of gaseous N2 and Ar are placed in separate flasks of equal volume at the same temperature. Tell whether each of the following statements is true or false. a. There are more molecules of N2 present than atoms of Ar. Blank 1 b. The pressure is greater in the Ar flask. Blank 2 c. The N2 molecules collide more frequently with the walls of the flask than do the Ar atoms.
Answers
Answer:
c. The N2 molecules collide more frequently with the walls of the flask than do the Ar atoms.
Explanation:
The statements are:
a. There are more molecules of N2 present than atoms of Ar. FALSE. Because 1 mol of molecules of N2 = 28g and 1 mol of molecules of Ar = 40g. As there are equal MASSES, you will have more molecules of N2 than Ar molecules
b. The pressure is greater in the Ar flask. FALSE
Because pressure is directly proportional to amount of molecules. As molecules N2 > Molecules Ar. The pressure is greater in N2 flask
c. The N2 molecules collide more frequently with the walls of the flask than do the Ar atoms. TRUE
The collision probability of N2 is higher because there are more molecules presents
please help me with these

Answers
1) Diesel has a higher viscosity than petrol.
2) Petrol is more flammable than diesel.
3) The formula will be C₁₀H₂₂.
4) The equation is; 2C8H18+25O2→16CO2+18H2O.
What is the hydrocarbon?Depending on the precise composition and temperature, the viscosity of gasoline and diesel can change. In general, diesel is more viscous than gasoline. Higher viscosity fluids are thicker and flow more slowly than lower viscosity fluids because viscosity relates to the resistance of a fluid to flow.
Diesel is less flammable than gasoline. The lowest temperature at which gasoline can evaporate and turn into an ignitable combination in air is known as its flash point, and it is lower for gasoline. Compared to diesel fuel, petrol vapors are much more flammable and can ignite at lower temperatures.
Learn more about hydrocarbon:https://brainly.com/question/31643106
#SPJ1
explain with the help of examples difference between elements and compounds
Answers
Answer:
Elements are pure substances which are composed of only one type of atom. Compound are substances which are formed by two or more different types of elements that are united chemically in fixed proportions. ... Some of the examples of elements are Iron, Copper, Gold, etc. A few examples of compounds are NaOH, NaCl, etc.
CuBr2 percent composition
Answers
The percent composition of CuBr₂ is approximately 28.46% of Cu and 71.54% of Br.
To determine the percent composition of CuBr₂ (copper(II) bromide), we need to calculate the mass of each element in the compound and then divide it by the molar mass of the entire compound.
The molar mass of CuBr₂ can be calculated by adding up the atomic masses of copper (Cu) and bromine (Br) in the compound. The atomic masses of Cu and Br are approximately 63.55 g/mol and 79.90 g/mol, respectively.
Molar mass of CuBr₂ = (63.55 g/mol) + 2(79.90 g/mol) = 223.35 g/mol
Now, let's calculate the percent composition of each element in CuBr₂:
Percent composition of copper (Cu):
Mass of Cu = (63.55 g/mol) / 223.35 g/mol × 100% ≈ 28.46%
Percent composition of bromine (Br):
Mass of Br = 2(79.90 g/mol) / 223.35 g/mol × 100% ≈ 71.54%
Therefore, the percent composition of CuBr₂ is approximately:
- Copper (Cu): 28.46%
- Bromine (Br): 71.54%
These values represent the relative mass percentages of copper and bromine in the compound CuBr₂.
for more such questions on composition
https://brainly.com/question/28250237
#SPJ8
Three elements have the electron configurations 1s2 2s2 2p6 3s2 3p6, 1s2 2s2 2p6 3s2, and
1s2 2s2 2p6 3s2 3p6 4s1. The first ionization energies of the three elements (not in the same order) are 0.4189, 0.7377, and 1.505 MJ/mol. The atomic radii are 1.60, 0.94, and 2.35 .Identify the three elements and match the appropriate values of ionization energy and atomic radius to each configuration.
Answers
Explanation:
First element
1s2 2s2 2p6 3s2 3p6
Atomic Number = 18
Element = Argon
Second Element
1s2 2s2 2p6 3s2
Atomic Number = 12
Element = Magnesium
Second Element
1s2 2s2 2p6 3s2 3p6 4s1
Atomic Number = 19
Element = Potassium
In the periodic table;
The general trend is for ionization energy to increase moving from left to right across an element period. It would decrease down the group.
Both Argon and Magnesium are on the same period. Potassium is o the period below.
This leads us to;
Magnesium - 0.7377 MJ/mol
Argon - 1.505 MJ/mol
Potassium - 0.4189 MJ/mol
The atomic radius of atoms generally decreases from left to right across a period. It would increase down the group.
This leads to;
Magnesium - 1.60
Argon - 0.94
Potassium - 2.35
A solution is made by dissolving 38.81 grams of nickel (II) sulfate, NiSO4, in enough water to make 0.467
liters of solution. Calculate the molarity of this solution.
Answers
The molarity of the NiSO₄ solution made by dissolving 38.81 grams of nickel (ii) sulfate, NiSO₄, in enough water to make 0.467 liters of solution is 0.535 M
How do i determine the molarity of the solution?First, we shall obtain the mole of 38.81 grams of nickel (ii) sulfate, NiSO₄. Details below:
Mass of NiSO₄ = 38.81 grams Molar mass of NiSO₄ = 154.75 g/molMole of NiSO₄ = ?Mole of NiSO₄ = mass / molar mass
= 38.81 / 154.75
= 0.25 mole
Now, we shall determine the molarity of the solution. Details below:
Mole of NiSO₄ = 0.25 moleVolume of solution = 10.467 LMolarity of solution = ?Molarity of solution = mole / volume
= 0.25 / 0.467
= 0.535 M
Thus, the molarity of the solution is 0.535 M
Learn more about molarity:
https://brainly.com/question/16073358
#SPJ1
What is the pH of 6.00 M H2CO3 if it has 7% dissociation? SHOW YOUR WORK!!!
Answers
From the calculation, the pH of the solution can be obtained as 0.39.
What is the pH of the solution?We first have to obtain the dissociation constant of the solution;
α = √Ka/C
Ka = Cα^2
Ka = 4.9 * 10^-3 * 6
Ka = 0.0294
Then we have that;
0.0294 = [x]^2/[6 - x]
0.0294 * [6 - x] = x^2
0.1764 - 0.0294 x = x^2
x^2 + 0.0294 x - 0.1764 =0
x = 0.41 M
Thus the pH of the solution is;
pH = -log[0.41]
= 0.39
pH is an important parameter in chemistry, biology, and many other fields, as it can affect the behavior and properties of substances and reactions.
Learn more about pH:https://brainly.com/question/15289714
#SPJ1
Enter the complementary strand of this DNA strand. Give your answer as a string.

Answers
Answer:
atagcca
Explanation:
t goes with a, and c goes with g
atagcca matches with
ta tcggt
_____ resources are resources that cannot be replenished within a lifetime.
Question 1 options:
Nonrenewable
Renewable
Living
Endangered
Answers
Nonrenewable natural resources are resources that cannot be replenished within a lifetime.
If 40.5 g of KCl are dissolved 250.0 g of water at 25.0 °C in an
insulated container, a temperature change is observed. The AH of
solution of KCI is 17.2 kJ/mol. Assuming that the specific heat of the
solution is 4.184 J/(g °C), and that no heat is gained or lost by the
container, what will be the final temperature of the solution?
Answers
The final temperature of the solution is 25.22 °C.
What is final temperature?The final temperature of a system depends on a variety of factors, such as the type of material, its mass, its volume, the amount of energy added or removed, and the type of environment it is in.
First, we need to calculate the amount of energy released from the KCl solution. This is equal to the moles of KCl multiplied by the AH of the solution.
Moles of KCl = 40.5/74.55 = 0.54 mol
Energy released = 0.54 mol * 17.2 kJ/mol = 9.288 kJ
Now, we need to calculate the amount of heat energy absorbed by the 250 g of water. This is equal to the mass of the water multiplied by the specific heat multiplied by the temperature change.
Heat absorbed = 250 g * 4.184 J/(g °C) * (T - 25 °C)
Since we know the amount of energy released, we can rearrange this equation to solve for the temperature change.
9.288 kJ = 250 g * 4.184 J/(g °C) * (T - 25 °C)
T - 25 °C = 9.288 kJ / (250 g * 4.184 J/(g °C))
T - 25 °C = 0.22 °C
Therefore, the final temperature of the solution is 25.22 °C.
To learn more about final temperature
https://brainly.com/question/22338034
#SPJ1
Give the systematic name for each of the following organic molecules and enter it in the space provided. Be sure to include appropriate punctuation.

Answers
Systematic name : 5-chloro-2-pentanol (or 5-chloropentan-2-ol)
Systematic name : 1,2-difluoro-3-heptanol (or 1,2-difluoroheptan-3-ol).
What is pentanol used for?The active site of numerous reactions is the hydroxyl group (OH). Pentyl butyrate, which has an apricot-like aroma, is the ester that results from the reaction of 1-pentanol and butyric acid. Amyl acetate, also known as pentyl acetate, is the ester that is created when 1-pentanol and acetic acid are combined.
A research evaluating the efficacy of diesel fuel blends with different amounts of pentanol as an additive was done in 2014. Higher pentanol concentrations resulted in higher gaseous emissions at the expense of lower particulate emissions.
Learn more about pentanol
https://brainly.com/question/30218582
#SPJ1
an imaginary gas phase reaction, according to the equation below is carried out in a closed container. 3.5 moles of A and 3.5 moles of B is introduced in the container. Reaction is carried out at 31.70C and volume of the container is 9.3 L. Percent yield of this reaction is 86.8%. Calculate the pressure of the container after reaction took place. R =0.0821 L.atm/mol.K
A+ 2B → AB2
Answers
bro hwnsbsjqjsnanqbsbjsjwhsshjs incorjssnsn
an expression is show below. - 1 2/3 - x
Assume that this expression is plotted on a number line starting at - 1 2/3
What could the value of x be in order to move right more than 3 units on the number line from the starting point? Select all t
hat apply.
Answers
Answer:
What’s the answer ♀️
Explanation:
need help with this question

Answers
Answer:
Sugar, Carbon Dioxide, And Water
Explanation:
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be simple substances, chemical compounds, or alloys. Chemical elements may or may not be included in the definition, depending on expert viewpoint.
Read image for instructions A heterogeneous mixture B moleculeC liquid D homogeneous mixture

Answers
ANSWER
Heterogeneous mixture
EXPLANATION:
Given information
From the above image, we can see that we have three elements named a, b, and c. The elements a and c are attached to each other while element b can move freely.
Recall that, the combination of two or more elements is called a compound.
The above image shows the combination of a compound and an element
Hence, we can say that it is a heterogeneous mixture
Which relationship is present in a solution that has a pH of 7
Answers
Answer:
neutral
Explanation:
pH is usually (but not always) between 0 and 14. Knowing the dependence of pH on [H +], we can summarize as follows: If pH < 7, then the solution is acidic. If pH = 7, then the solution is neutral
Calculate the amount of energy in kilojoules needed to change 207 g
of water ice at −
10 ∘C
to steam at 125 ∘C
. The following constants may be useful:
Cm (ice)=36.57 J/(mol⋅∘C)
Cm (water)=75.40 J/(mol⋅∘C)
Cm (steam)=36.04 J/(mol⋅∘C)
ΔHfus=+6.01 kJ/mol
ΔHvap=+40.67 kJ/mol
Answers
Therefore, the amount of energy required to change 207 g of water ice at −10 ∘C to steam at 125 ∘C is 744.3618 kJ.
What does kJ mean in terms of energy?Similar to how kilometres measure distance, a kilojoule is a measurement used to measure energy. Some nations continue to use the Calories (Cal) system, which was once used to quantify food energy. These are the conversions: 1 kJ equals 0.2 Cal.
To figure out how much energy is needed to convert 207 g of water ice at -10°C to steam at 125°C, we must divide the process into several stages and figure out how much energy is needed for each one:
Heating ice from -10°C to 0°C:
q1 = m × Cm(ice) × ΔT
= 207 g ÷ 18.02 g/mol × 36.57 J/(mol⋅∘C) × (0 - (-10)) ∘C
= 41324.8 J
= 41.3248 kJ
Melting ice at 0°C:
q2 = n × ΔHfus
= m ÷ M × ΔHfus
= 207 g ÷ 18.02 g/mol × 6.01 kJ/mol
= 56.804 kJ
Heating water from 0°C to 100°C:
q3 = m × Cm(water) × ΔT
= 207 g ÷ 18.02 g/mol × 75.40 J/(mol⋅∘C) × (100 - 0) ∘C
= 174667.6 J
= 174.6676 kJ
Vaporizing water at 100°C:
q4 = n × ΔHvap
= m ÷ M × ΔHvap
= 207 g ÷ 18.02 g/mol × 40.67 kJ/mol
= 467.7326 kJ
Heating steam from 100°C to 125°C:
q5 = m × Cm(steam) × ΔT
= 207 g ÷ 18.02 g/mol × 36.04 J/(mol⋅∘C) × (125 - 100) ∘C
= 3832.8 J
= 3.8328 kJ
Total energy required:
qtotal = q1+q2+q3+q4+q5
= 41.3248 kJ + 56.804 kJ + 174.6676 kJ + 467.7326 kJ + 3.8328 kJ
= 744.3618 kJ.
To know more about energy visit:-
https://brainly.com/question/8630757
#SPJ9
Petra dropped her beaker on the floor. She placed her gloves on the table. She then started to pick up the small pieces with her hand and place them in the broken-glass container. At the end of lab, she told her instructor that there was broken glass on the floor. The teacher swept it up and placed the glass in the broken-glass container.
Aside from dropping the beaker, how many errors did Petra commit?
one
two
three
four
Answers
Answer:
three
Explanation:
Answer:
Three
Explanation:
1.Dropping said beaker
2.Removing her gloves
3.Picking up the item with her hands exposed
If this was a crime then she would have gotten her fingerprints all over the beaker and since its glass it should not be too hard to identify as a fingerprint.
Hope this helps
Dont forget to smash that heart at the bottom! <3
Plz mark brainliest
Have a great day, You’re amazing!
An energy of 4.50x10^-19 J/photon was released when an electron drops to a lower energy state, what is the wavelength of the photon? What color does this energy correspond to?
Answers
4.41 × 10⁻⁴⁵m is the wavelength of the photon and the energy correspond to red color.
What do you mean by the wavelength ?The term wavelength is defined as the distance between two identical points that are adjacent crests and troughs.
The SI unit of wavelength is metre mostly represented as m.
The wavelength is mostly represented by λ is the Greek letter lambda.
Given:
E = 4.50x10⁻¹⁹ J/photon
h = Planck constant = 6.626 × 10⁻³⁴
ν = ?
E = hν
ν = E/h
By substituting the values in above question and we get,
= 4.50x10^-19 / 6.626 × 10⁻³⁴
= 0.679 × 10⁻¹⁵
c = 3 × 10⁸
E = hc/λ
λ = hc/E
By substituting the values in above question and we get,
λ = 6.626 × 10⁻³⁴ × 3 × 10⁸ / 4.50x10⁻¹⁹
λ = 4.41 × 10⁻⁴⁵m
Thus, the wavelength of the photon is 4.41 × 10⁻⁴⁵m and color does this energy correspond to red.
To learn more about the wavelength, follow the link;
https://brainly.com/question/13533093
#SPJ9
Of the following regions of the electromagnetic spectrum, which one has the shortest wavelength?
a.
gamma rays
b.
infrared
c.
radio waves
d.
X rays
e.
microwaves
f.
ultraviolet
Answers
Answer:
A ---->gamma ray
Explanation:
Gamma rays have the highest frequencies among all electromagnetic waves and therefore have the shortest wavelengths.
Need help with this two part question

Answers
The ideal gas law and stoichiometry must be used to calculate the volume of carbon dioxide gas produced by the breakdown of 4.09 g of calcium carbonate at STP (Standard Temperature and Pressure).
Use the molar mass of calcium carbonate (CaCO3) to determine how many moles it contains. CaCO3 has a molar mass of 100.09 g/mol.
CaCO3 mass divided by its molar mass equals the number of moles of CaCO3: 4.09 g/100.09 g/mol.
The number of moles of carbon dioxide (CO2) generated may be calculated using the stoichiometric ratio from the balancing equation. By using the equation:
A unit of CaCO3 and CO2 is produced.
CO2 moles equal the same number of moles of CaCO3.
Use the ideal gas law to translate the volume of carbon dioxide into moles.
Learn more about CaCO3 at :
https://brainly.com/question/29792038
#SPJ1
The composition of a compound is 28.73% K, 1.48% H, 22.76% P, and 47.03% O. The molar mass of the
compound is 136.1 g/mol.
I
Answers
The compound has an empirical formula of \(K_2H_2P_2O_8\) and a molecular formula of \(K_2HPO_4\).
The given compound has a percent composition of K = 28.73%, H = 1.48%, P = 22.76%, and O = 47.03%. Its molar mass is 136.1 g/mol. To determine its molecular formula, we need to find its empirical formula and calculate its molecular formula from its empirical formula.The empirical formula is the smallest whole number ratio of atoms in a compound. It can be determined by converting the percent composition of the elements into their respective moles and dividing each by the smallest number of moles calculated. The moles of K, H, P, and O in 100 g of the compound are: K = 28.73 g x (1 mol/39.1 g) = 0.734 molH = 1.48 g x (1 mol/1.01 g) = 1.46 molP = 22.76 g x (1 mol/30.97 g) = 0.736 molO = 47.03 g x (1 mol/16.00 g) = 2.94 molDividing each by the smallest number of moles gives the following ratios: K = 0.734/0.734 = 1H = 1.46/0.734 = 2P = 0.736/0.734 = 1.002O = 2.94/0.734 = 4. The empirical formula of the compound is \(K_2H_2P_2O_8\). To calculate the molecular formula, we need to determine the factor by which the empirical formula should be multiplied to obtain the molecular formula. This can be done by comparing the molar mass of the empirical formula to the molar mass of the compound.The molar mass of \(K_2H_2P_2O_8\) is: \(M(K_2H_2P_2O_8)\) = (2 x 39.1 g/mol) + (2 x 1.01 g/mol) + (2 x 30.97 g/mol) + (8 x 16.00 g/mol) = 276.2 g/mol. The factor by which the empirical formula should be multiplied is: M(molecular formula)/M(empirical formula) = 136.1 g/mol/276.2 g/mol = 0.4935. The molecular formula is obtained by multiplying the empirical formula by this factor: \(K_2H_2P_2O_8 * 0.4935 = K_2HPO_4\). Therefore, the molecular formula of the compound is \(K_2HPO_4\).The molecular formula of the given compound having a composition of 28.73% K, 1.48% H, 22.76% P, and 47.03% O with a molar mass of 136.1 g/mol is \(K_2HPO_4\). The empirical formula of the compound is \(K_2H_2P_2O_8\). The compound's molecular formula is calculated by determining the factor by which the empirical formula should be multiplied to obtain the molecular formula. The factor is M(molecular formula)/M(empirical formula) = 136.1 g/mol/276.2 g/mol = 0.4935. The molecular formula of the compound is obtained by multiplying the empirical formula by this factor, resulting in the molecular formula \(K_2HPO_4\).For more questions on empirical formula
https://brainly.com/question/13058832
#SPJ8
The correct question would be as
The composition of a compound is 28.73% K. 1.48% H, 22.76% P, and 47.03% O. The molar mass of the compound is 136.1 g/mol. What is the Molecular Formula of the compound?
\(KH_2PO_4\\KH_3PO_4\\K_2H_4P_20_{12}\\K_2H_3PO_6\)
what electrical energy can be transforme
d when we switch on the electrical bulb
Answers
The electrical energy can be transformed when we switch on the electrical bulb is light and heat energy. The correct option is B.
What is electrical energy?Electrical energy is the energy produced when electrons move from one point to another in a tube. The movement is of charged particles. They conduct electricity and constitute a current.
In electrical energy, heat energy is transformed into light energy. This happens because the rods of wire become red-hot and heat is produced.
Thus, the correct option is B. light and heat energy.
To learn more about electrical energy, refer to the below link:
https://brainly.com/question/14241903
#SPJ1
The question is incomplete. Your most probably complete question is given below:
A, sound energy
B, light and heat energy
C. Light and sound energy
D. Chemical and sound energy