A solution is prepared by adding 500 ml of 0. 3 m naclo to 500 ml of 0. 4 m hclo. what is the ph of this solution?
Answers
The pH of a solution can be determined by the concentration of hydrogen ions (H+) present in the solution. In this case, we need to find the pH of a solution prepared by adding 500 ml of 0.3 M NaClO to 500 ml of 0.4 M HClO.
To find the pH of the solution, we first need to determine the concentration of H+ ions in the solution. Since both NaClO and HClO are strong electrolytes, they will dissociate completely in water.
NaClO dissociates into Na+ and ClO- ions, while HClO dissociates into H+ and ClO- ions.
Now, let's calculate the concentration of H+ ions in the final solution.
For NaClO:
The given volume of NaClO is 500 ml, and the concentration is 0.3 M.
Therefore, the number of moles of NaClO = volume x concentration = 0.5 L x 0.3 M = 0.15 moles.
Since NaClO dissociates into Na+ and ClO- ions in a 1:1 ratio, the concentration of ClO- ions is also 0.15 M.
For HClO:
The given volume of HClO is also 500 ml, and the concentration is 0.4 M.
Therefore, the number of moles of HClO = volume x concentration = 0.5 L x 0.4 M = 0.2 moles.
Since HClO dissociates into H+ and ClO- ions in a 1:1 ratio, the concentration of H+ ions is also 0.2 M.
Now, we add the concentrations of H+ ions from both NaClO and HClO:
0.15 M (from NaClO) + 0.2 M (from HClO) = 0.35 M
Therefore, the concentration of H+ ions in the final solution is 0.35 M.
To find the pH, we can use the formula:
pH = -log[H+]
Substituting the value of [H+] = 0.35 M into the formula:
pH = -log(0.35)
Calculating this, we find that the pH of the solution is approximately 0.46.
Please note that the pH scale ranges from 0 to 14, with 7 being neutral. A pH below 7 is acidic, while a pH above 7 is basic. In this case, the pH is below 7, indicating that the solution is acidic.
To know more about hydrogen ions visit:-
https://brainly.com/question/8069088
#SPJ11
Related Questions
Which formula represents a covalent compound
A: NaCl
B: CaO
C: MgS
D: H2O2
Answers
Answer:
Answer is D dont forget to mark btainlist
Answer:
\(\boxed {\boxed {\sf D. \ H_2O_2}}\)
Explanation:
A covalent compound is a molecule made up of covalent bonds, where the atoms share pairs of valence electrons. These compounds consist of two or more nonmetals.
Essentially, if a compound contains a metal, it is an ionic or metallic compound, not a covalent compound.
A. NaCl
This is sodium chloride. It contains sodium (Na) and chlorine (Cl). Sodium is a metal, so this is ionic.
B. CaO
This is calcium oxide. It contains calcium (Ca) and oxygen (O). Calcium is a metal, so this is ionic.
C. MgS
This is magnesium sulfide. It contains magnesium (Mg) and sulfur (S). Magnesium is a metal, so this is ionic.
D. H₂O₂
This is hydrogen peroxide. It contains hydrogen (H) and oxygen (O). Both of these elements are non-metals, so this is a covalent compound.
The correct choice is D. H₂O₂
[URGENT!] - Equilibrium Problem
Consider the following reaction:
4A + 2B ⇌ 2C + D (all gases at STP)
Initially, the reaction system is at equilibrium, with [A] = [B] = 2.60 M, and [C] = [D] = 3.10 M, making K = 0.0964. The reaction takes place in a 5.00-liter container. Moles of B are removed until the new equilibrium concentration of C is 2.70. How many moles of B were removed?
Answers
The equilibrium constant expression for this reaction is:
K = [C]2[D] / [A]4[B]2
We can rearrange this expression to solve for [B]:
[B] = √([A]4[C]2[D] / K)
Plugging in the given values, we get:
[B] = √((2.60 M)4(2.70 M)2(3.10 M) / 0.0964)
= √(6.76 M)
= 0.26 M
The initial concentration of B is 2.60 M, so the change in concentration of B is 2.60 M - 0.26 M = 2.34 M.
Since the reaction takes place in a 5.00-liter container, the number of moles of B that were removed is 2.34 M * 5.00 L = 11.7 moles.
Therefore, 11.7 moles of B were removed.
why solids do not undergo diffusion
Answers
Answer:
Solids do not undergo diffusion because it has a fixed structure (vibrates in a fixed position) and it also cannot be compressed.
Hoped this helped in any way
Have a nice day
HELPPPPP PLZZZ ASAPPPPPPPP

Answers
Sulfuric acid decomposes into sulfur trioxide gas and water. What would be the respective coefficients for balancing?.
Answers
Chemical equations must be balanced, which requires that the quantity and type of atoms on both sides of the reaction arrow be equal.
What are the balanced reaction's coefficients?As a result, chemical equations must be balanced, which requires that the quantity and type of atoms on both sides of the reaction arrow be equal. Coefficients are the values added in front of formulas to balance equations; they multiply each atom in a formula.The number in front of the variable phrases is the coefficient, just like in algebra. The number in front of the formula is the coefficient in chemistry.
Sulphur trioxide = SO_3
Sulfuric acid = H_2SO_4
SO 3(g) + H2O (l) ---> H2SO4 (aq)
To learn more about balanced reaction's refer to:
https://brainly.com/question/26694427
#SPJ4
Lead has a density of 11.4 g/cm^3. What is the density in kilograms per cubic meter?
Answers
The density in kg/m³ = 1.14 x 10⁴
Further explanationDensity is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
With the same mass, the volume of objects that have a high density will be smaller than objects with a smaller type of density
The unit of density can be expressed in g/cm³ or kg/m³
Density formula:
\(\large {\boxed {\bold {\rho ~ = ~ \frac {m} {V}}}}\)
ρ = density , g/cm³ or kg/m³
m = mass , g or kg
v = volume , cm³ or m³
A density of Lead : ρ = 11.4 g/cm³
the density in kg/m³ :
\(\tt 11.4~\dfrac{g}{cm^3}\times \dfrac{kg}{10^3~g}\times \dfrac{cm^3}{10^{-6}~m^3}=\boxed{\bold{1.14\times 10^4~\dfrac{kg}{m^3}}}\)
Cuales son los tipos de yemas
Answers
axilar, cuando está ubicada en la axila de una hoja (también denominadas laterales);
adventicia, cuando ocurre en los demás lugares, por ejemplo en el tronco o en las raíces.
One similarity between B and Si is the explosive combustion of their hydrides in air. Write balanced equations for the combustion of B₂H₆ and of Si₄H₁₀.
Answers
The balanced equations for the combustion are:
Combustion of B2H6 : B2H6(g) + 3O2(g) → B2O3(s) + 3H2O(g) Combustion of Si4O10 : Si4O10(g) + 13O2(g) → SiO2(s) + 10H2O(g)
A fuel (the reductant) and an oxidant, typically atmospheric oxygen, undergo a high-temperature exothermic redox chemical reaction that results in oxidized, frequently gaseous products and a mixture known as smoke. Since a flame only appears when substances undergoing combustion evaporate, combustion does not always result in fire, but when it does, a flame is a distinctive sign of the event.
Fuel combustion reactions release usable thermal energy as they occur (heat). Most of the cars we drive are powered by combustion reactions, and a large portion of our electricity is produced by them as well.
Learn more about Combustion here: https://brainly.com/question/15117038
#SPJ4
Determine the resulting pH when 0.040 mol of solid NaOH is added to a 200.0 mL buffer containing 0.100 mol C6H5NH3Cl and 0.500 M C6H5NH2. The value of Kb for C6HNH2 is 4.3 × 10-10.
Answers
The pH of the solution is 5.4.
This is a basic buffer problem. The reaction in the buffer is:
C6H5NH3+ (aq) + H2O (l) ↔ C6H5NH2 (aq) + H3O+ (aq)
The Kb expression for the weak base C6H5NH2 is:
Kb = [C6H5NH2][H3O+] / [C6H5NH3+]
We can assume that the initial concentration of C6H5NH3+ and C6H5NH2 is equal to their original concentrations. Let x be the amount of H3O+ formed by the reaction. Then the new concentration of C6H5NH3+ is (0.100 - x) mol/L and the new concentration of C6H5NH2 is (0.500 + x) mol/L.
Now, we can set up the Kb expression and solve for x:
4.3 × 10-10 = [(0.500 + x)(x)] / (0.100 - x)
Solving this equation gives x = 3.76 × 10-6 M.
This means that the new concentration of H3O+ is 3.76 × 10-6 M, and the new pH is:
pH = -log[H3O+] = -log(3.76 × 10-6) ≈ 5.4
Therefore, the resulting pH when 0.040 mol of solid NaOH is added to the buffer is approximately 5.4.
To know more about titration please visit:
https://brainly.com/question/31271061
#SPJ11
Sodium nitrate and ammonium chloride are soluble in
water. Sodium chloride and ammonium nitrate are also
soluble in water. What ions will be present in a solution
that results when solutions of sodium nitrate and
ammonium chloride are mixed
Answers
When sodium nitrate (NaNO3) and ammonium chloride (NH4Cl) are mixed in water, they will dissociate into their respective ions:
NaNO3 → Na+ + NO3-
NH4Cl → NH4+ + Cl-
What is a homogeneous mixture?A homogeneous mixture is a type of mixture that has a uniform composition and appears visually and chemically the same throughout its entire volume. In other words, a homogeneous mixture is one that has the same proportion of components in all parts of the mixture.
All of these ions are soluble in water. Therefore, when the two solutions are mixed, the resulting solution will contain all four ions: Na+, NO3-, NH4+, and Cl-.
There will be no chemical reaction between the ions from the two salts as they do not react with each other. Thus, the resulting solution will be a homogeneous mixture of the four ions.
To know more about homogeneous mixture, visit:
https://brainly.com/question/24898889
#SPJ1
What is the formula for sulfate?
What is the charge of sulfate?
What is the correct formula for copper (II) sulfate?

Answers
Answer:
SO₄²-, -2, CuSO4
Explanation:
Dang that was long.
Choose the paramagnetic species from below.
Ar
O
Ti4+
All of the above are paramagnetic.
None of the above are paramagnetic.
Answers
The correct answer is option (c) Ti4+.
The species which are attracted to a magnetic field are known as paramagnetic species. If we talk about the given options, then we can see that there are only 3 species that are given. Out of these three, only Ti4+ is paramagnetic. How can we determine whether a species is paramagnetic or not? The species which contain unpaired electrons are paramagnetic in nature. If there are all paired electrons, then the species are diamagnetic. If we talk about Ti4+, then it contains 2 unpaired electrons, which makes it paramagnetic. This is the reason why the correct answer is Ti4+.In Ar, all the electrons are paired, which makes it diamagnetic. In O, there are 2 unpaired electrons, which makes it paramagnetic. How can we determine whether a species is paramagnetic or not? The species which contain unpaired electrons are paramagnetic in nature. If there are all paired electrons, then the species are diamagnetic.
Learn more about paramagnetic species at brainly.com/question/29990302
#SPJ11
Which one of the following will dissociate into the largest number of ions in solution?
A) LiuSO4
B) KI
C) LIC.H.02
D) Na PO
E) CH2O
Answers
The most ions will form from the dissociation of \(Na_{3}PO_{4}\) in solution.
A dissociation reaction is a chemical process in which a molecule splits into two or more other components. The molecules separate or fragment into smaller particles like atoms, ions, or radicals during this reversible reaction.
Simply put, a dissociation reaction is a chemical process in which a molecule is divided into at least two separate components or compounds.
The dissociation reaction has the following formula: AB \(\rightarrow\) A + B.
In water, Na3PO4 dissolves and separates into its ions. Phosphate and sodium ions make up its ions.
To sum up, the chemical equation that illustrates this transition is:
\(Na_{3}PO_{4}(s) \rightarrow 3Na + PO^{3-}_{4}(aq)\)
Learn more about dissociation reaction:
brainly.com/question/14671105
#SPJ4
What kind of mixture is the copper chloride and water ? Please explain !!!!!!!!!!!!!!!!!!!!! Will mark brainliest !!!!!!!!!!!!!!!!!!!!!!
Answers
Answer:
Copper(II) chloride is the chemical compound with the chemical formula CuCl2. This is a light brown solid, which slowly absorbs moisture to form a blue-green dihydrate. Both the anhydrous and the dihydrate forms occur naturally as the very rare
Explanation:
if photons of light with a wavelength of 189.1 nm illuminate the surface of a metal, what will the kinetic energy of the electron be, in j? (note: this question depends on your answer to part 1. your final answer to this question must be submitted after you've submitted your final answer to part 1)
Answers
The kinetic energy of the electron be, in j is 4.1766 × 10⁴⁹.
Kinetic energy is the power an object has because of its motion. If we want to boost up an object, then we must follow a force. making use of force requires us to do paintings. After work has been accomplished, energy has been transferred to the object, and the object might be moving with a new consistent speed.
Calculation:-
wavelength = 189.1 nm
= 189.1 × 10⁻⁹ m
c = λf
f = 189.1 × 10⁻⁹ m / 3 × 10⁸
= 63.033 × ⁻ ¹⁷
kinetic energy = hf
= 6.626 × 10⁻³⁴ × 63.033 × ⁻ ¹⁷
= 417.66 × 10⁻⁵¹
= 4.1766 × 10⁴⁹
The kinetic energy of an item is the electricity that it possesses because of its motion. it is defined because the work needed to boost up a frame of a given mass from relaxation to its stated pace. Having gained this energy for the duration of its acceleration, the frame maintains this kinetic electricity except for its pace changes.
Potential energy saved energy that relies upon the relative position of numerous components of a gadget. A spring has greater potential energy when it is compressed or stretched. A steel ball has extra capability strength raised above the ground than it has after falling to Earth.
Learn more about kinetic energy here:-https://brainly.com/question/25959744
#SPJ4
Which of the following elements is most reactive.
A Chlorine
B Bromine
C Fluorine
D Helium
Answers
Answer:
c.
Fluorine
Explanation:
Some isotopes of an atom are ____________ , while others are ____________ , or radioactive. Carbon ____________ is an example of an unstable isotope of carbon.
Answers
Some isotopes of an atom are stable, while others are unstable or radioactive. Carbon-14 is an example of an unstable isotope of carbon.
Isotopes are variants of an element that have the same number of protons but different numbers of neutrons. While some isotopes of an atom are stable and do not undergo spontaneous changes, others are unstable and undergo radioactive decay.
Radioactive isotopes have an imbalance in the ratio of protons to neutrons, leading to an unstable atomic nucleus. To reach a more stable state, these isotopes release energy in the form of radiation by undergoing radioactive decay. This decay process can involve the emission of alpha particles, beta particles, or gamma rays.
Carbon-14 is a specific example of an unstable isotope of carbon. It has 6 protons and 8 neutrons, which makes it slightly heavier than the stable carbon isotope, carbon-12. Carbon-14 is radioactive and undergoes beta decay, where a neutron in the nucleus converts into a proton, releasing a beta particle (an electron) in the process.
Therefore, some isotopes of an atom are stable, while others are unstable or radioactive. Carbon-14 is an example of an unstable isotope of carbon.
For more details regarding isotopes, visit:
https://brainly.com/question/28039996
#SPJ12
why does the benzaldehyde starting material not form an enolate
Answers
Benzaldehyde does not form an enolate because it lacks an alpha-hydrogen, which is essential for enolate formation. In most carbonyl compounds, the alpha-hydrogen is adjacent to the carbonyl group (C=O) and can be deprotonated by a strong base.
This deprotonation leads to the formation of an enolate ion, which is stabilized by resonance with the carbonyl group. However, in the case of benzaldehyde, the carbonyl group is directly attached to a benzene ring. The alpha position does not have a hydrogen atom but rather, it is connected to the aromatic ring. Since there is no alpha-hydrogen to deprotonate, benzaldehyde cannot form an enolate. This characteristic of benzaldehyde makes it behave differently in reactions compared to other carbonyl compounds, such as aldehydes and ketones. It is important to consider the absence of an alpha-hydrogen in benzaldehyde when predicting or analyzing its reactivity in various chemical reactions.
To know more about Benzaldehyde
https://brainly.com/question/30230005
#SPJ11
which if the following questions can be answered by science?
Answers
Answer:
Need the choices before i can give answers thanks
Explanation:
5. True or False: A growth rate can be only positive and negative, it can never be zero.....
A. True
B. False
Answers
Answer:
True
Explanation:
What is the major threat to the ogallala aquifer? a:too much extraction and not enough recharge b:too much recharge and not enough extraction c:farms switching crops that are raised and harvested d:installation of water conservation devices
Answers
The major threat to the Ogallala Aquifer is a: too much extraction and not enough recharge.
The Ogallala Aquifer is a vital source of water for agriculture and communities in the Great Plains region of the United States. However, due to increased demand and unsustainable practices, such as excessive pumping for irrigation, the aquifer is being depleted faster than it can naturally recharge. This imbalance between extraction and recharge is causing the water levels in the aquifer to decline rapidly. If this continues, it could lead to long-term consequences, such as reduced water availability, land subsidence, and increased costs for pumping. It is crucial to find sustainable water management strategies and promote water conservation practices to protect the Ogallala Aquifer for future generations.
Know more about agriculture here:
https://brainly.com/question/31113136
#SPJ11
If the reactants on the left side of a chemical equation are c3h8 + 5o2, the products in a balanced equation could be.
Answers
To determine the number of atoms of each element we need to multiply stoichiometry that is written in front of the molecule to the number that is written on the foot of the element. Hence, the balanced chemical for the combustion of C₃H₈ is C₃H₈ + 5O₂ → 3CO₂ + 4H₂O.
Balanced chemical equation
Balanced equation is defined as the reaction where the number of atoms of each species is same on reactant and product side. In balanced equation mass can neither be destroyed nor be created.
The skeletal equation can be written as:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
The number atoms of C on reactant and product side is 3 so C is balanced.
The number of atoms of O is 10 on reactant and on product side it is 10. So, O is balanced.
The number of atoms of H is 8 on reactant and on product side it is 8. So, H is balanced.
Hence, the balanced chemical equation for the combustion of C₃H₈ is given as:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Learn more about balanced chemical reaction from the link given below.
https://brainly.com/question/7105418
#SPJ4
why should care be exercised in preparation of column to prevent air bubbles from being trapped in adsorbent
Answers
By exercising care in column preparation and preventing air bubbles from being trapped in the adsorbent, you can achieve more accurate and efficient chromatographic separations.
What are the effects of column preparation on the analysis of samples?
Care should be taken during column preparation because trapped air bubbles can lead to several issues, such as:
1. Decreased column efficiency: Air bubbles can create voids or channels in the adsorbent, disrupting the uniform flow of the mobile phase and reducing the separation efficiency of the column.
2. Poor peak resolution: Trapped air bubbles can cause peak broadening and tailing, making it difficult to accurately identify and quantify individual compounds in the mixture.
3. Longer analysis time: Inefficient separation due to air bubbles can increase the time required for the analysis, leading to longer experimental procedures and potentially increased costs.
To avoid these issues, follow these steps when preparing a column:
1. Choose the appropriate adsorbent and particle size for your specific application.
2. Slowly add the adsorbent slurry into the column to minimize the chance of trapping air bubbles.
3. Gently tap the column to encourage air bubbles to rise to the surface.
4. Allow the column to settle and recheck for air bubbles, repeating the process if necessary.
By exercising care in column preparation and preventing air bubbles from being trapped in the adsorbent, you can achieve more accurate and efficient chromatographic separations.
To know more about Column Preparation:
https://brainly.com/question/31040810
#SPJ11
Which description is an example of the effects of rising temperatures?
Answers
The mass of .10 moles of iron is:
Answers
Answer:
so mass in gram=560grams
Explanation:
number of moles=10moles
molar mass=56grams/moles
mass in gram of Fe=?
as we know that
\(number of moles=\frac{mass in gram}{molar mass}\)
evaluating the formula
number of moles×molar mass=mass in gram
mass in gram=10moles×56grams/moles
mass in gram=560grams
i hope this will help you :)
Name a substance that is made of individual atoms instead of moleules and explain why
Answers
Elements: HE,NE,AR,KR,XE,RN, and OG
2) How much heat in kJ is
required to boil 50.0 grams of
water?
Answers
Answer:
The heat required to boil water is called the heat of vaporization, and it is the amount of energy required to change the state of a substance from a liquid to a gas at a constant temperature. The heat of vaporization for water is approximately 40.7 kJ/mol or 2257 J/gram.
To calculate the heat required to boil 50.0 grams of water, you would multiply the heat of vaporization (2257 J/g) by the number of grams of water:
Heat (J) = 2257 J/g x 50 g
Heat (J) = 112,850 J
To convert Joules to kilojoules (kJ), divide by 1000.
Heat (kJ) = 112,850 J / 1000 = 112.85 kJ
Therefore, it takes 112.85 kJ of heat to boil 50.0 grams of water.
How many quarts of 5% solution can be made from 4.73 grams of
drug?
Answers
The number of quarts of 5% solution that can be made from 4.73 grams of the drug is 100 quarts.
To calculate the number of quarts of 5% solution that can be made from 4.73 grams of the drug, we need to use the formula that relates the amount of drug to the concentration and volume of the solution. Let's first convert the drug quantity to grams. Since 1 gram is equivalent to 1000 milligrams, then:
4.73 grams = 4730 milligrams
Now, let's plug in the values into the formula and solve for the volume of the solution.
Amount of drug (in grams) = Concentration (as a decimal) × Volume of solution (in milliliters)
To convert milliliters to quarts, we will divide the volume by 946.35 (1 quart = 946.35 milliliters). So we have:
4730 mg = 0.05 × Volume of solution (in milliliters)
Volume of solution = 4730 ÷ 0.05 = 94,600 milliliters (ml)
Number of quarts of solution = 946.35 = 100 quarts (rounded to the nearest whole number).
Therefore, 100 quarts of 5% solution can be made from 4.73 grams of the drug.
Learn more about quarts: https://brainly.com/question/4418837
#SPJ11
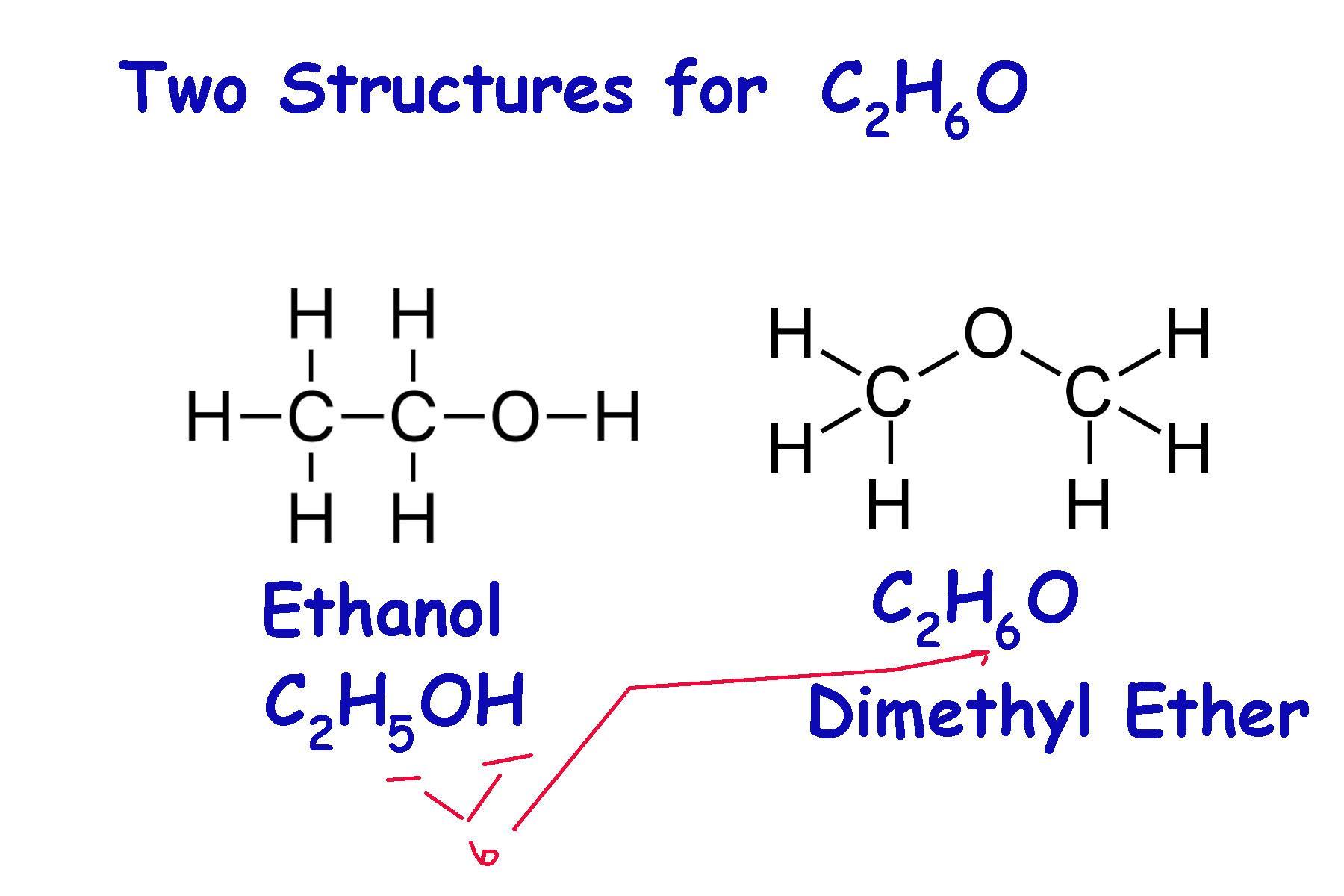
(3) What is the chemical formula of this molecule? Η Η | | H—C-C-0-HHHWhat is the name of this molecule? Build it in the playground.
Answers
Answer:
\(C_{2}\)\(H_{6}\)O This can be one of two componds: ethanol or dimethyl ether.
Explanation:
I'm having trouble interpreting:
Η Η | | H—C-C-0-HHH
The manner in which a chemical structure is written plays a large roll in how to interpret what compund it is. In this case we can count 2 carbons, 1 oxygen, and 6 hydrogens.
From this, we are tempted to quickly conclude and write:
\(C_{2}\)\(H_{6}\)O
and state that this is clearly ethanol. Why would it be anything else?
Although this is a correct possible interpretation of the information, but \(C_{2}\)\(H_{6}\)O it has at least two different structures: ethanol and dimethyl ether. See the attached diagram.
Most combination of elements can have more than 1 structure, and are therefore different compounds. Because if this, the manner in which the structure is drawn is essential to how it is interpreted. Even the formula notation has certain rules designed to help identify the compound. The attached diagram illustrates the two ways ethanol and dimethyl ether can be lav=belled: C2H5OH, which highligts the -OH group and identifies it as an alcohol. Dimethyl ether has the O atom at the end, without the H atom stuck behind it. This signals that it is incorporated somewhere else in the molecule.
find the average speed of a person who swims 105 m in 70 s
Answers
Answer:
1.5 m/s
Explanation:
105 / 70 = 1.5