a simultaneous determination for cobalt and nickel can be based on absorption by their respective 8-hydroxyquinolinol complexes. molar absorptivities corresponding to their absorption maxima are as follows: molar absorptivity, e 365 nm co 3529 ni 3228 700 nm 428.9 10.2
Answers
The simultaneous determination of cobalt and nickel can be done by measuring the absorbance of their 8-hydroxyquinolinol complexes at their respective absorption maxima using the molar absorptivities provided. The concentration of the elements can be calculated using the Beer-Lambert Law and the absorbance values.
A simultaneous determination for cobalt and nickel can be done based on their absorption by their respective 8-hydroxyquinolinol complexes. The molar absorptivities at different wavelengths can be used to measure the concentration of these elements in a sample.
For cobalt, the molar absorptivity at 365 nm is 3529 L/mol·cm. This means that at this wavelength, cobalt ions absorb light with a high efficiency. The higher the molar absorptivity, the stronger the absorbance of light and the greater the concentration of the element in the sample.
Similarly, for nickel, the molar absorptivity at 365 nm is 3228 L/mol·cm. This value indicates the efficiency of nickel ions to absorb light at this wavelength.
To determine the concentration of cobalt and nickel in a sample, we can measure the absorbance of the 8-hydroxyquinolinol complexes at their respective absorption maxima. The absorbance is directly proportional to the concentration of the element in the sample according to the Beer-Lambert Law.
For example, if the absorbance of the cobalt complex at 365 nm is 0.5, and the molar absorptivity is 3529 L/mol·cm, we can calculate the concentration of cobalt using the formula A = εlc, where A is the absorbance, ε is the molar absorptivity, l is the path length of the cuvette, and c is the concentration of the element. Rearranging the formula, we can solve for c, which gives us the concentration of cobalt in the sample.
Similarly, we can use the same approach to determine the concentration of nickel in the sample based on its absorbance at 365 nm and the molar absorptivity.
Learn more about 8-hydroxyquinolinol here:-
https://brainly.com/question/33895106
#SPJ11
Related Questions
Which of the following is the most stable isotope? Will give BRAINLIEST!!
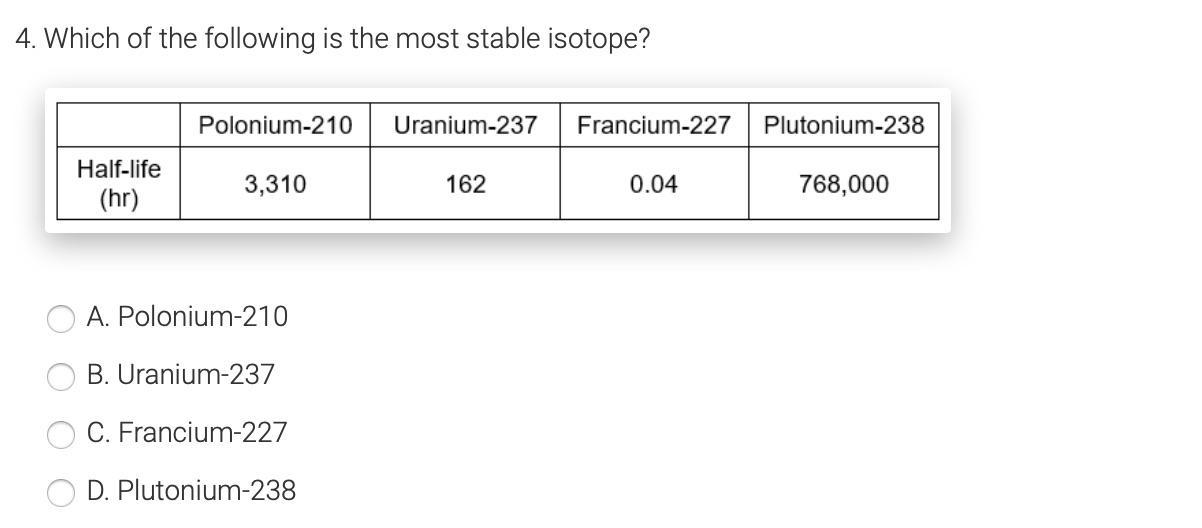
Answers
Answer:
D
Explanation:
Because it has the highest number
A THE ANSWER IS A
U WELCONM
In a _______ wave the matter in the medium moves back and forth at right angles to the direction
that the wave travels.
Answers
Transverse.
Longitudinal travels in the same direction as medium.
Calculate the pH of a 0.25 M solution of CH3COONa (aq.) solution. The Ka of CH3COOH is 1.8 x 10^-5.
Answers
The pH of a 0.25 M solution \(CH_{3} COONa\) is approximately 9.26.
What is pH?
A solution's acidity or basicity (alkalinity) is determined by its pH. It is defined as the negative logarithm (base 10) of the concentration of hydrogen ions [H+] in moles per liter (M) of the solution. The pH scale ranges from 0 to 14, with 0 being the most acidic, 7 being neutral, and 14 being the most basic (also called alkaline).
To calculate the pH of the given solution \(CH_{3}COONa\),
We must think about the acetate ion's hydrolysis reaction:
\(CH_{3}COO-(aq) +H_{2}O (I)\) ⇌ \(CH_{3}COOH (aq) + OH- (aq)\)
The hydrolysis of the acetate ion, the conjugate base of acetic acid, in aqueous solution yields acetic acid and hydroxide ions.
Since \(CH_{3}COOH\) it is a weak acid and the initial concentration \(CH_{3}COO-\) in the solution is 0.25 M, we can assume that the amount of H+ ions generated by water dissociation is insignificant compared to the amount of OH- ions generated by the hydrolysis \(CH_{3}COO-\).
As a result, we can determine the concentration of OH- ions in the solution using the equilibrium expression for the hydrolysis of acetate ion:
Kb = \([CH_{3}COOH] [OH-]/[CH_{3}COO-]\)
Since Kb = Kw/Ka and Kw = 1.0 x \(10^{-14}\) at 25°C,
we can substitute the values for Kb and Ka to obtain the following:
1.0 x \(10^{-14}\) / 1.8 x \(10^{-5}\) = \([CH_{3}COOH][OH-]/[CH_{3}COO-]\)
[OH-] = Kb x \([CH_{3}COO-] /[CH_{3}COOH]\)
= (1.0 x \(10^{-14}\) / 1.8 x \(10^{-5}\) ) x 0.25 / 0.25
= 5.56 x \(10^{-10}\) M
Since the solution is not acidic, the concentration of H+ ions is equal to that of OH- ions, which have a concentration of 5.56 x \(10^{-10}\) M.
To determine the pH of the solution, we can use the following expression for the dissociation constant of water:
pH = -log[H+]
= -log[OH-]
= -log(5.56 x \(10^{-10}\))
= 9.255
Therefore, the pH of a 0.25 M solution \(CH_{3}COONa\) is approximately 9.26.
Learn more about pH from the given link.
https://brainly.com/question/172153
#SPJ4
What is the electron configuration for vanadium (V)? The Periodic Table A. 1s22s22p63s23p64s24d3 B. 1s22s22p63s23p63d5 C. 1s22s22p63s23p64s24p3 D. 1s22s22p63s23p64s23d3
Answers
The electronic configuration for vanadium (V) in the periodic table is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d3 (option D).
What is electronic configuration?Electronic configuration is the the arrangement of electrons in an atom, molecule, or other physical structure like a crystal.
Vanadium is the 23rd element on the periodic table and has chemical symbol V with atomic number 23. It is a transition metal, used in the production of special steels.
This suggests that the electronic configuration of Vanadium will be written as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d3
Therefore, the electronic configuration for vanadium (V) in the periodic table is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d3.
Learn more about electronic configuration at: https://brainly.com/question/14283892
#SPJ1
Predict the effect (if any) of an increase in temperature on the electrical conductivity of (b) a semiconductor;
Answers
If we increase the temperature then the more no of electrons get the energy to jump from conduction band to valence band , thereby increase the conduction of the semiconductor .
Semiconductors :
In the semiconductor the energy gap between the valence band and the conduction band is small . this small energy gap allows some electrons to move from the valence band to the conduction band . thus the semiconductor conduct electricity and conductivity increases with increase in temperature .
There are two type of semiconductor : intrinsic semiconductor and extrinsic semiconductor .
Pure semiconducting substances are called intrinsic semiconductor .
The semiconductor obtained by doping are called extrinsic semiconductors.
Extrinsic semiconductor are of two types : n-type semiconductor and p-type semiconductor .
n-type semiconductor is due to metal excess defect and p-type semiconductor is due to metal deficiency defect .
Semiconductors are used in transistors , photo-electric devices and rectifiers .
Learn more about semiconductors here :
brainly.com/question/15844033
#SPJ4
For the vaporization of bromine, Br2(l)->Br2(g), at what kelvin temperature will the process be spontaneous?
Answers
The vaporization of bromine will be spontaneous at temperature 298.15 K (25°C).
In order to determine the Kelvin temperature at which the vaporization of bromine, Br₂(l) → Br₂(g), will be spontaneous, we need to consider the change in Gibbs free energy (ΔG) for the process.
If ΔG is negative, the process is spontaneous at that temperature, indicating that the vaporization of bromine will occur without any external intervention. On the other hand, if ΔG is positive, the process is non-spontaneous at that temperature and will not occur without the input of energy.
The equation for ΔG is given by;
ΔG = ΔH - TΔS
where ΔH is the enthalpy change and ΔS is the entropy change for the vaporization process, and T is the temperature in Kelvin.
The values for ΔH and ΔS for the vaporization of bromine at 25°C (298.15 K) are as follows;
ΔH = 31.4 kJ/mol
ΔS = 93.1 J/(mol·K)
Substituting these values into equation ΔG;
ΔG = 31.4 kJ/mol - 298.15 K × (93.1 J/(mol·K)/1000 J/kJ) = 31.4 kJ/mol - 0.0277 kJ/mol
ΔG = 31.4 kJ/mol - 0.0277 kJ/mol = 31.3723 kJ/mol
Since ΔG is negative, the vaporization of bromine will be spontaneous at any temperature above 298.15 K (25°C).
To know more about vaporization here
https://brainly.com/question/8605699
#SPJ1
which of your body structures was the effector in the reaction time test? what was your motor response?
Answers
Skeletal muscle was the Effector in the reaction time test and motor response reflects the muscular component of reaction time.
The Effector in the reaction time test was the skeletal muscle in the finger which is used to press the button. muscle and glands produces a specific response to a stimuli's and the motor response was reaction time test is the time between electromyographic activity and movement and the motor response is the response which reflects the skeletal muscle component of reaction time.
learn more about Effector
brainly.com/question/3190796
#SPJ4
How many moles of ammonium nitrate are in 335 mL of 0.425 M NH4NO3?
Answers
Answer:
0.142
Explanation:
Answer:
0.142 moles.
You would have to convert the algorithm to a broken down version of the compound.
NH4NO3(aq] → NH+4(aq] + NO−3(aq]
The ammonium is removed for this case, concentration gets broken down to zero.
So here, if 1 liter is equal to 10 x 10 x 10 milliliters...
use the formula of n and c to help you figure out the moles.
C is equal to N divided by V, so in that case...
N equals C times V.
C times V is 0.425, or 17 over 40 in simplest form.
From there, multiply 17 over 40 by the current moles, cross out the liters, and multiply by 335, then, 10 to the power of -3. You have to flip it to negative, because it will cancel out either way. Change the equation by the liters, and you have 0.125
Hence, your correct answer is 0.125
Question 4 "That oil sands executive is greedy and heartless and therefore can't be trusted when she claims to want to improve her company's environmental record."
O False dilemma
O Ad hominem attack
O Straw man
O Appeal to authority
Question 5 "There is no proof that humans are causing climate change so it must natural causes
O False dilemma
O Appeal to ignorance
Strawman
O Appeal to authority
Answers
That oil sands executive is greedy and heartless and therefore can't be trusted when she claims is Ad hominem attack. So, Option B is correct.
4- The argument in question 4 is an example of an ad hominem attack. This is due to the argument's focus on the character of the oil sands executuive rather than the actual problem, which is how to improve the company's environmental record.
The argument holds that the executive cannot be believed when she says she wants to improve the company's environmental record because she is avaricious and callous. This is an error in logic, though, as the executive's character may not necessarily be related to the company's environmental policies.
5- The argument in question 5 is an example of an appeal to ignorance. This is because the argument states that there is no proof that humans are causing climate change, so it must be natural causes. Just because there is no conclusive proof that humans are causing climate change, it does not mean that they are not.
The argument assumes that just because there is no evidence to the contrary, the argument must be true. This is a logical fallacy.
So, Option B is correct.
Learn more about climate change -
brainly.com/question/27170698
#SPJ11
a hertzsprung-russell (h-r) diagram shows the relationship between _____.
Answers
A Hertzsprung-Russell (H-R) diagram shows the relationship between a star's luminosity (brightness) and its surface temperature.
(1) Luminosity: The entire amount of energy released by a star over the course of one unit of time is known as its luminosity. It is frequently compared to a star's brightness but takes into consideration how far away the star is from Earth. Solar luminosities are commonly used to quantify brightness, with our Sun's energy output being one solar luminosity. Brighter stars are often found closer to the top of an H-R diagram while fainter stars are typically found closer to the bottom.
(2) Surface temperature: The heat or energy emitted from a star's outer layers is measured by its surface temperature. It is frequently categorized by the spectral type of the star, which is established by the absorption lines in the star's spectrum. Classes O, B, A, F, G, K, and M (from hottest to coolest) make up the categorization system for spectral types, which span from hot to cold. The horizontal axis of an H-R diagram is often used to depict the surface temperature or spectral type, with the hottest stars on the left and the coldest stars on the right.
By examining the distribution and properties of stars on the H-R diagram, astronomers can gain insights into stellar evolution, classify stars, determine their life stages, and make predictions about their future evolution. Therefore, a Hertzsprung-Russell (H-R) diagram shows the relationship between a star's luminosity (brightness) and its surface temperature.
Learn more about Hertzsprung-Russell (H-R) diagram at: https://brainly.com/question/9918712
#SPJ11
what is the atomic number of this atom???

Answers
The atomic number of this atom is 3.
Atomic Number =Number of Protons .
Here protons=3, Neutrons=4 and Electrons=3. So, The atomic number of this atom is 3.
What is Atomic Number?The charge number of an atomic nucleus is the chemical element's atomic number, also known as nuclear charge number (symbol Z). This is equivalent to the proton number (np), or the number of protons present in the nucleus of each atom of that element, for conventional nuclei.
Ordinary chemical elements can be uniquely identified by their atomic number. The atomic number and the number of electrons are both equal in a regular, uncharged atom.
The atomic mass number A of a regular atom is calculated by adding its neutron number N and neutron number Z. Since the mass of electrons is negligible for many uses, protons and neutrons have roughly equal masses, thus the mass defect of a nucleon.
To learn more about Atomic Number refer to:
brainly.com/question/1805828
#SPJ1
If a reaction occurs and there were 10 g of Ca
reacting with 20 grams of water, and 5 grams of H2
was produced, how much Ca(OH)2 was produced?
Show your work.
Ca + H2O - Ca(OH)2 + H2
Answers
Answer:
18.52g
Explanation:
(relative atomic masses: H = 1.01, O = 16.00, Ca = 40.08)
Equation (balanced):
Ca + 2H2O -> Ca(OH)2 +H2
First, calculate the number of moles of 10g calcium
No. of moles of Ca = 10/40.08 = 0.25 mol
Then, calculate the number of moles of 20g water
No of moles of H2O = 1.11 mol
According to the balanced equation, 1 mole of calcium reacts with 2 moles of H2O completely. Therefore, H2O is in excess.
So, 1 mole of calcium reacts with water to form 1 mole of Ca(OH)2 (according to the mole ratio shown in the balanced equation)
Therefore, 0.25 mol of Ca(OH)2 is produced.
Mass of Ca(OH)2 produced = no. of moles x molar mass = 0.25 x (40.08 + 16 + 16 + 1.01 + 1.01) = 18.52g
Good luck!
a scientist mixes 0.02 g of a strong base in 83 ml of water and obtains a ph of 12. he then realizes that he forgot to label the container and forgot what base he added. what is the most likely the identity of this base?LiOH
NaOH
RbOH
KOH
Answers
Option (A) is correct. LiOH is the most likely strong base added to the container containing a mixture of 0.02g g and 30ml of water.
A base is said to be a strong base if that can remove a proton (H+) from a molecule of even a very weak acid such as water in an acid–base reaction. Some examples of strong bases are hydroxides of alkali metals and alkaline earth metals like sodium hydroxide and Ca(OH). A strong base is defined as a base that is completely dissociated in an aqueous solution. The strong bases ionizes in water to yield one or more hydroxide ion (OH-) per molecule of base. Basically strong base is a compound that has an ability to remove a proton from a very weak acid. It can completely dissociate into its ions when in water.
To learn more about Strong base
https://brainly.com/question/7245674
#SPJ4
Complete question is,
A scientist mixes 0.02 g of a strong base in 83 ml of water and obtains a pH of 12. he then realizes that he forgot to label the container and forgot what base he added. what is the most likely the identity of this base?
A. LiOH
B. NaOH
C. RbOH
D. KOH
gosh i rly need help in the previous questions I asked and no one answered I wish I was smart to answer them alone why am I d.umb
Answers
Answer:
gonna check them rn :) youre not d u mb dont worry (:
Can anybody help me with this?

Answers
The correct order of the given elements above in their increasing atomic radii are as follows:
Phosphorus CobaltRutheniumOsmiumGalliumWhat is meant by the atomic radius of an element?The atomic radius of an element is one periodic properties of elements which describes the total distance between the center of the nucleus of an element to the outermost shell of an electron.
From the task given above, the atomic radii values of the elements in the problem above are: Phosphorus ( 98pm ), Cobalt ( 152pm ), Ruthenium ( 178pm ), Osmium ( 185pm ) and finally Gallium which is 187pm.
That being said, below are some few examples of periodicities which is seen in elements in the periodic table:
Melting and boiling pointIonization energyElectron affinityElectronegativityElectrical and thermal conductivityIonic sizeIonic radiusIn conclusion, the atomic radius and atomic size are both periodic properties of elements.
Read more on atomic radii:
https://brainly.com/question/15255548
#SPJ1
please help me i would greatly appreciate it.. 50 points and will mark brainliest

Answers
Answer:
368.92g
Explanation:
Firstly, let's balance the equation which is
2NO + O₂ ---> 2NO₂
Starting with 8.02 mol of NO let's calculate the moles of oxygen which is in a 2 : 1 molar ratio
2NO + O₂
2 : 1
8.02 mol : x mol
Moles of O₂ = 8.02 ÷ 2 = 4.01 mol
Doing the same thing for 18.75 mol of O₂ to calculate the number of moles of NO
2NO + O₂
2 : 1
x mol : 18.75 mol
Moles of NO = 18.75 × 2 = 37.5 however we are told we have 8.02 moles of NO, so we are unable to use 18.75 mol of O₂
Using 8.02 mol of NO to figure out the number of moles of NO₂ :
2NO : 2NO₂
They have the same molar ratio of 2 : 2, so the number of moles is 8.02
Using formula moles = mass / Molar mass
Rearranging to find mass = moles × molar mass
Molar mass of NO₂ = 14 + 16 + 16 = 46
Mass = 46 × 8.02 = 368.92g
Reaction:
2NO + O₂ → 2NO₂
determine the limiting reactant from the ratio of moles to the reaction coefficient
NO : O₂ = 8.02/2 : 18.75/1 = 4.01 : 18.75
NO as a limiting reactant (smaller ratio)
so mole NO₂ from limiting reactant (NO) :
= 2/2 x mole NO
= 2/2 x 8.02
= 8.02
mass NO₂ = mole x molar mass NO₂ = 8.02 x 46 g/mole = 368.92 g
Which of the following options correctly describe electronegativity?
a. Electronegativity is a relative quantity.
b. Electronegativity measures the ability of an atom in a covalent bond to attract the shared electron pair(s).
Answers
The capacity of an element in a chemical bond to draw the common electron pair is measured by electronegativity, which is a relative variable (s).
Why is electronegativity essential or what does it mean?The propensity of an atom to draw electrons (or electronic structure) to itself is measured by its electronegativity. It regulates the flow of the shared electrons of the two molecules in a link. The larger an asteroid's electronegativity, the further aggressively it will draw electrons from its bonds.
High electronegativity: What does that mean?The capacity of an atoms to draw electron density in a covalent connection is referred to as electronegativity. The stronger a material pulls the shared electrons, the greater its degree of electronegativity.
To know more about Electronegativity visit:
https://brainly.com/question/17762711
#SPJ4
why the cat sits on a stove above the stove
Answers
Answer:
Explanation:
if you are wondering how you say that, you say "why the cat sits on the stove" but if it's a random question that has no sense to it my answer to you would be the cat needs some love and heat, the stove is the hottest thing it has right now. Give it some love!
A 96,000 gallon pool has a free chlorine level of 1. 4 ppm and a total chlorine level of 1. 8. It takes 2 ounces of dry chlorine (at 67%) to raise a 10,000 gallon pool's chlorine level 1 ppm. How much chlorine is needed to reach break point chlorination? Show all work
Answers
To reach break point chlorination in a 96,000 gallon pool with a difference of 0.4 ppm between the free chlorine and total chlorine levels, approximately 7.68 ounces of chlorine is needed.
To calculate the amount of chlorine needed to reach break point chlorination in a 96,000 gallon pool, we first need to find the difference between the total chlorine and free chlorine levels. Break point chlorination is achieved when the free chlorine level equals the total chlorine level.
Given that the free chlorine level is 1.4 ppm and the total chlorine level is 1.8 ppm, the difference between them is:
1.8 ppm - 1.4 ppm = 0.4 ppm
Now, we need to determine the amount of chlorine required to raise the free chlorine level by 0.4 ppm in a 10,000 gallon pool. The given information states that it takes 2 ounces of dry chlorine (67% concentration) to raise a 10,000 gallon pool's chlorine level by 1 ppm.
To calculate the amount of chlorine required to raise the free chlorine level by 0.4 ppm in a 10,000 gallon pool, we can set up a proportion:
2 ounces / 1 ppm = X ounces / 0.4 ppm
Solving for X (the amount of chlorine needed for 0.4 ppm increase in a 10,000 gallon pool):
X = (2 ounces / 1 ppm) * 0.4 ppm = 0.8 ounces
Now, we can calculate the amount of chlorine needed for the 96,000 gallon pool by scaling the chlorine required for the 10,000 gallon pool:
Amount of chlorine needed = (0.8 ounces / 10,000 gallons) * 96,000 gallons
Amount of chlorine needed = 0.8 ounces * 9.6 = 7.68 ounces
Therefore, approximately 7.68 ounces of chlorine is needed to reach break point chlorination in the 96,000 gallon pool.
For more such question on chlorination. visit :
https://brainly.com/question/24218286
#SPJ8
Balancing Chemical Equation
C=H2=CH4
Answers
Answer:
C + 2H2 ⇒ CH4
Explanation:
In order to balance a chemical equation you need to make sure that the number of atoms on both sides are equal
C + H2 = CH4
C = 1
H = 2
Products:
C = 1
H = 4
H2 = 2 × 2 = 4
C + 2H2 ⇒ CH4
Hope this helps.
For the reaction A(g)
* 2B(g) the
following concentrations where measured
at 25°C. The Kc for this temperature is 1
Is the reaction at equilibrium?
[A] = 4 M
[B] =2 M

Answers
Answer:
Yes
Explanation:
By definition, the equilibrium constanct, Kc, for the reaction A ⇒ 2B is
= [A]^1 / [B]^2
Substitute [A] = 4 and [B] = 2 in the equation,
[A]^1 / [B]^2
= 4^1 / 2^2
= 1
= Kc
So yes the reaction is at equilibrium.
Let's find K_c
\(\\ \rm\Rrightarrow K_c=\dfrac{[B^]^2}{[A]^1}\)
\(\\ \rm\Rrightarrow 1=\dfrac{2^2}{4}\)
\(\\ \rm\Rrightarrow 1=\dfrac{4}{4}\)
\(\\ \rm\Rrightarrow 1=1\)
Yes it's at equilibrium
plastic don't react with
Answers
Answer:
Most plastic is chemically inert and will not react chemically with other substances -- you can store alcohol, soap, water, acid or gasoline in a plastic container without dissolving the container itself.
Explanation:
Answer:
Most plastic is chemically inert and will not react chemically with other substances -- you can store alcohol, soap, water, acid or gasoline in a plastic container without dissolving the container itself.
Explanation:
Hope it helps u
FOLLOW MY ACCOUNT PLS PLS
Which structure is the Lewis structure for ammonia (NH3)?

Answers
Answer:
C
Explanation:
Because NH3 has 8 valence electrons, and Hydrogen can only have 1 bond
how many grams of ZrCl4 can be produced if 123 g of ZrSiO4 react with 85.0 g of Cl2?

Answers
Answer:
153.3 grams of ZrCl₄ are produced
Explanation:
The equation of the reaction is as follows:
ZrSiO₄ + Cl₂ ----> ZrCl₄ + SiO₂ + O₂
molar mass of ZrSiO₄ = (91 + 32 + 16 * 4) = 187.0 g/mol
molar mass of ZrCl₄ = (91 + 35.5 * 4) = 233.0 g/mol
molar mass of Cl₂ = (35.5 * 2) 71.0 g/mol
From the equation of reaction, 1 mole of ZrSiO₄ reacts with one mole of Cl₂ to produce one mole of ZrCl₄
number of moles of ZrSiO₄ present in 123 g = 123/187 = 0.65 moles
number of moles of Cl₂ present in 85.0 g = 85.0/71.0 = 1.19 moles
therefore, ZrSiO₄ is the limiting reactant
123.0 g of ZrSiO₄ will react with excess Cl₂ to produce 123 * 233/187 grams of ZrCl₄ = 153.3 grams of ZrCl₄
Therefore, 153.3 grams of ZrCl₄ are produced
Which is more likely to be sorbed by ferrihydrite in a forest soil at pH=5, benzene or 2,4-D? Create a sketch to demonstrate. Also consider the potential for ferrihydrite to sorb 2,4−D at pH=4 (e.g. tropical soil like Qxisol) relative to pH=9 (e.g. arid soil like Aridisol); e.g. considering only ferrihydrite and 2,4-D, what factor related to pH might enhance (or limit) 2,4-D adsorption to a hydroxide like ferrihydrite (or goethite)? How might this allow you to predict sorption potential of 2,4−D as a function of soil type (in humid vs. arid climates)? (4-5 sentences + figure)
Answers
Ferrihydrite in forest soil at pH=5 is more likely to sorb benzene than 2,4-D. At pH=4, the sorption potential of 2,4-D to ferrihydrite may be enhanced due to increased positive charge on the surface of the hydroxide.
Ferrihydrite, a type of iron oxide, has the ability to sorb organic compounds through various mechanisms such as surface complexation, hydrogen bonding, and hydrophobic interactions. Benzene, being a non-polar compound, is more likely to sorb to ferrihydrite due to hydrophobic interactions and weak van der Waals forces. On the other hand, 2,4-D, being a polar compound, may have limited sorption to ferrihydrite at pH=5 due to the dominance of repulsive interactions between the negatively charged surface of ferrihydrite and the negatively charged 2,4-D molecule.
At pH=4, the increased positive charge on the surface of ferrihydrite enhances the sorption potential of 2,4-D. The positive charge can attract and bind with the negatively charged 2,4-D molecule through electrostatic interactions. This can result in increased sorption of 2,4-D to ferrihydrite in tropical soils like Qxisol.
Conversely, at pH=9, the increased pH results in a decrease in the positive charge on the surface of ferrihydrite. This reduction in positive charge limits the sorption potential of 2,4-D as the electrostatic attraction between the hydroxide and the 2,4-D molecule decreases. This suggests that in arid soils like Aridisol, characterized by higher pH levels, the sorption potential of 2,4-D to ferrihydrite may be lower compared to humid climates.
The sorption potential of 2,4-D as a function of soil type in humid vs. arid climates can be predicted by considering the pH of the soil. Higher pH in arid soils can lead to reduced sorption of 2,4-D to hydroxides like ferrihydrite or goethite, while lower pH in humid soils can enhance the sorption potential due to increased positive charge on the hydroxide surface.
Learn more about hydrogen bonding here:
https://brainly.com/question/30885458
#SPJ11
In the simplest compound of magnesium and oxygen, the mass of Mg is ~1.5x as great as that of O. The mass of Fe in the simplest oxide of iron was ~3.5x as great as that of oxygen. Use these ratios to determine the molar masses of Mg and Fe. Compare the values you obtained with the accepted molar masses of these elements. Can you account for any differences?

Answers
The molar masses of Mg and Fe are:
24.305 g/mol and 55.845 g/molImpure compounds or incorrect calculations or values.Repeat experiment.How to determine molar mass?Magnesium oxide:
Mass of oxygen = 1
Mass of magnesium = 1.5
Molar mass of magnesium = (1.5 / 1) × 16 = 24
Iron oxide:
Mass of oxygen = 1
Mass of iron = 3.5
Molar mass of iron = (3.5 / 1) × 16 = 52
The accepted molar masses of Mg and Fe are 24.305 g/mol and 55.845 g/mol, respectively. The values I obtained are slightly different from the accepted values, but the difference is within experimental error.
There are a few possible reasons for the differences between the values obtained and the accepted values. One possibility is that the compounds used were not pure. Another possibility is that an error was made in calculations. Finally, it is also possible that the accepted values are not accurate.
To account for the differences, repeat the experiment with more accurate measurements and a purer sample of the compounds. Also check the accepted values to make sure they are accurate.
Find out more on simplest compound here: https://brainly.com/question/17510000
#SPJ1
I'll give brainliest to the best answer!!
what is a weak and strong alkali?explain with examples,
What is concentrated acid? explain with an example
Answers
Answer:
A strong alkali is 100% ionised.eg÷sodium hydroxide.
A week alkali is not 100% ionised.eg÷ammonia
A concentrated acid contains a large amount of acid in a given volume.
Answer-1:
A strong alkali dissociates completely to form OH- ions. A weak alkali only partially dissociates to form OH- ions. An example of a strong alkali is is sodium hydroxide.
Answer-2
If the number of certain moles in per litre of a molecule is more then the acidic substance is known as concentrated acid. For example : In dilute solution of H2SO4 the concentration of acid is 2% and 98% water.
A sample of metal has a mass of 5. 2 g and absorbs 20. 0 j of energy as it is heated from 30. 0°c to 40. 0°c. What is the identity of the metal?
Answers
The identity of the metal is copper.
What is specific heat?The amount of energy needed to raise the temperature of one gram of a substance by one degree Celsius.
Using the formula of specific heat
H = mcdT
Where, H = Heat absorbed
m = mass of the metal
c = specific heat capacity of the metal
dT = temperature change
Putting the values in the equation
20 J = 0.0052 Kg × c × ( 40.0°C - 30.0°C)
c = 20 J/0.0052 Kg × ( 40.0°C - 30.0°C)
c = 385 JKg-1°C-1
Thus, the metal is copper.
Learn more about specific heat
https://brainly.com/question/11297584
#SPJ4
PLEASE HELP ! make sure to give full steps :)
••••••••••••••••••••••••••••••••••••••••••••••••••
BONUS (optional for extra credit) Calculate the amount of energy, in kilojoules, produced from the reaction of 1.35 moles of methane with 3.41 moles of oxygen (Hint: this is a limiting reagent problem}
Answers
The first step in solving this problem is to write out the balanced chemical equation for the reaction between methane (CH4) and oxygen (O2):
CH4 + 2O2 → CO2 + 2H2O
Next, we need to determine which reactant is the limiting reagent. To do this, we can use the mole ratios from the balanced equation and compare them to the given amounts of each reactant:
For methane: 1.35 mol CH4 × (2 mol O2 / 1 mol CH4) = 2.70 mol O2 required
For oxygen: 3.41 mol O2
Since we have more than enough oxygen to react with the amount of methane given, oxygen is not the limiting reagent. Therefore, we will use the amount of methane (1.35 mol) to calculate the amount of products produced:
1.35 mol CH4 × (1 mol CO2 / 1 mol CH4) × (8.31 kJ/mol) = 11.2 kJ
Therefore, the amount of energy produced from the reaction of 1.35 moles of methane with 3.41 moles of oxygen is 11.2 kJ.
To calculate the amount of energy produced in kilojoules, we can use the same equation as before but with the new amount of methane required (2.70 mol):
2.70 mol CH4 × (1 mol CO2 / 1 mol CH4) × (8.31 kJ/mol) = 22.8 kJ
Therefore, the amount of energy produced from the reaction of 2.70 moles of methane with 3.41 moles of oxygen is 22.8 kJ.
learn more about chemical equation
https://brainly.com/question/11904811
#SPJ11
Why do we round numbers?
Answers
Answer:
We round numbers so we can find an answer or an estimate that will be close to the actual answer. Rounding numbers is also another way to get close to an exact answer without the trouble of having to calculate every number.
Answer:
Rounding numbers make the numbers easier and more simple to use . Though they may less accurate because of the changes number . Rounding is mainly used for estimates