Answers
Answer:
in 10°C volume is 6 than in 20°C that is 12 and 30°C is 18 40°C is 24 and 50°C is 30
so answer is 30
Related Questions
The magnitude of one Kelvin, one Celsius degree, and one degree on the absolute temperature scale is the same. true or false . please explain it .....
Answers
Answer:
false
Explanation:
I don't think centigrade ranges from 0 to 100 and kelvin 237 and 373 absolute -273°c and 0K
Answer:
false............................
Which statement describes the conditions in which diamonds form?
Answers
Answer:
The answer to the question, 'which statement describe the formation of diamonds is option C. Diamond deposits are formed in the earth mantle and release to the earth surface by mean of volcanic eruptions which produce kimberlites. The formation of diamonds require very high temperatures and pressures. These conditions exist below the earth surface at the depth of about 150 kilometers and 1050 degree Celsius temperature.
Explanation:
Answer:
Diamond deposits are formed in the earth mantle and release to the earth surface by mean of volcanic eruptions which produce kimberlites
Explanation:
Whoever gets it right gets brainliest!!

Answers
When different atoms join together, they are called a compound. Compounds are made of molecules (so molecules could be an alternate answer, but not all molecules are compounds)
Answer:
it is compound
Explanation:
Two or more elements combined into one substance through a chemical reaction, such as water, form a chemical compound. All compounds are substances, but not all substances are compounds.
Help me as soon as possible I’m gonna dieeee
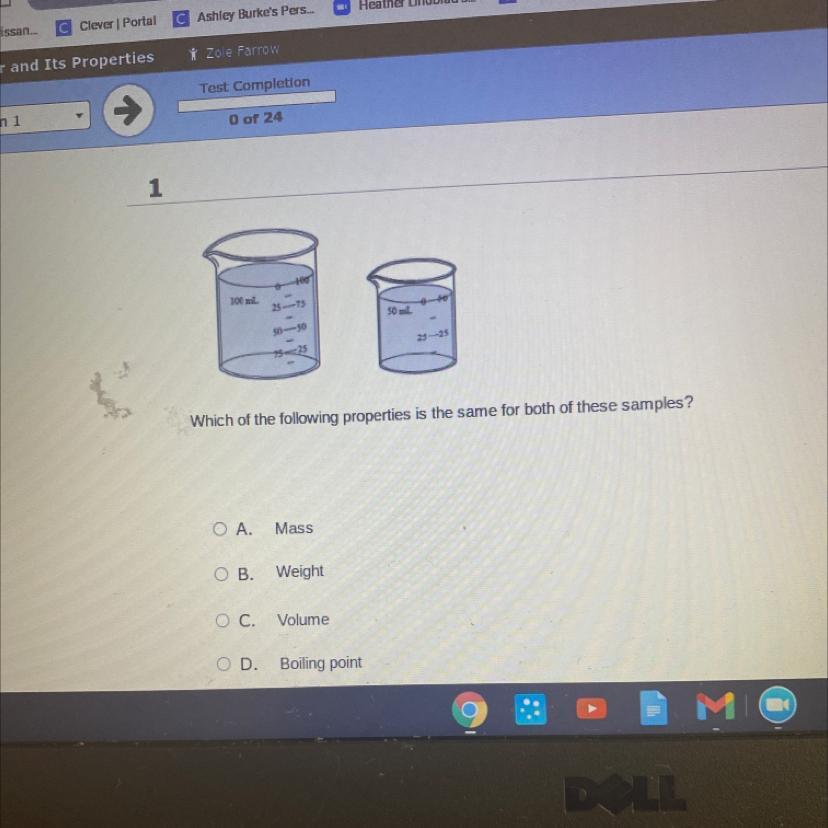
Answers
true or false: chemical reactions are usually written left to right
Answers
it is always reaction-> product
H2+O-> H2O
Type the correct answer in the box. The density is 1.26grams/ centimeters. How many pounds/foot is this? Use the conversion rates of 454 grams/1 pound and 28,317 centimeters/1 ft.. Express your answer to the correct number of significant figures.
Answers
Answer: 78.59 lb/ft
Explanation:
use stoichiometry to solve
1.26g/cm x ( 1pound/ 454 grams) X (28317 Cm/1 ft) = 78.59 pounds/ft
3. A helium filled balloon has a volume of 39.0mL at 17C and the current atmospheric pressure is reading 802mmHg on the barometer. What volume will the helium gas take up when the pressure reading on the barometer drops to 637mmHg and the temperature plummets to 7C?
Answers
Answer:
.0466 L
Explanation:
To start off, use dimensional analysis to convert everything to standard PV=nRT units.
\(\frac{39.0}{1}\) x \(\frac{1}{1000}\) = .039 L
\(\frac{802}{1}\) x \(\frac{1}{760}\) = 1.055 atm
17 +273 = 290
...and so on.
Solve for n for the starting pressure, volume and temp. You'll need it for the final equation.
n= PV/RT \(\frac{1.055 *.039}{.0821 * 290}\)= .0017 number of moles
Solve for volume of the end point.
V= nRT/P \(\frac{.0017*.0821*280}{.838}\) = .0466 L of helium, or 46.6ml Less pressure means it will take up more space.
Imagine that you are a scientist working in a very dry desert environment. This location has been experiencing a terrible drought, so there has been very little rainfall over the last few years. The drought is affecting the local farms, especially the farms that grow corn. You are asked to help choose a variety of corn that will grow the best in these drought conditions.
Which of the four varieties of corn that you tested in the lab would you choose?
Sunburst Variety
Golden Kernel Variety
Chok Full 'O Goodness Variety
Cob-o-Rama Variety
Why do you think this variety is the best choice?
Answers
Based on the information provided, I would recommend the Sunburst Variety of corn as the best choice for growing in the drought conditions of the desert environment.
What is the corn about?Sunburst Variety of corn has been specifically developed to be drought-tolerant, meaning that it can survive and produce a good yield even with minimal rainfall.
Therefore, I would recommend the Sunburst variety of corn as the best choice for growing in the desert environment due to its drought-tolerance, deep root system, heat tolerance, high yield, and good quality grain.
Learn more about corn from
https://brainly.com/question/397421
#SPJ1
select the correct iupac name for the alkane shown.multiple choice question.2-methyl-5-sec-butylhexane 2-ethyl-3,6-dimethylheptane 3,4,7-trimethyloctane 2,5,6-trimethyloctane
Answers
The IUPAC name for the alkane shown is 3-ethyloctane.
Define IUPAC name ?IUPAC name (International Union of Pure and Applied Chemistry name) is a standardized system for naming organic chemical compounds. The IUPAC naming system is based on a set of rules that specify how to name compounds based on their molecular structure, including the type and number of atoms, and their arrangement in the molecule. The IUPAC name provides a unique and unambiguous identifier for a specific chemical compound and helps to avoid confusion in communication between chemists and scientists. The IUPAC system is widely used in the scientific community and is recognized internationally as the standard system for naming organic compounds.
The IUPAC name for the alkane shown is 2-methyl-5-sec-butylhexane.
To learn more about IUPAC name follow the given link:
https://brainly.com/question/29405997
#SPJ1
Complete question:

PHMWMHWPTYSM(15 points)

Answers
Answer:
it's an example of a closed circuit
What is the difference between an ionic and molecular (covalent) bonds? Be sure to discuss the types of elements required, how electrons are involved, and give an example for each type of compound.
Answers
Explanation:
Here, we want to discuss ionic and covalent compounds
Ionic compounds also called electrovalent compounds are compounds that are formed when electrons are transferred from the valence shell of an electropositive atom (usually a metal) to the valence shell of an electronegative atom (usually a non-metal or a less electropositive or more electronegative atom), which results in bonding.
Take for example the case of Sodium and chlorine
Sodium has three shells with shell configuration 2,8,1 with one electron in its valent shell
Removing the extra 1 electron in its last shell will make it achieve the stability of a noble gas which is one of the reasons we do not have some elements existing in uncombined forms. It needs an atom to accept this extra electron it wants to remove
Now, here comes chlorine with shell configuration 2, 8, 7
It needs an extra electron to achieve the noble gas configuration
Hence, it will gladly accept the elcctron sodium wants to donate
This type of bonding that react from the transfer of the electron is the ionic bond. The electron is completely transferred to the chlorine shell and has no interaction anymore with the sodium atom
Example of this compound is Sodium Chloride
Molecular compounds are formed from electron sharing. The key word here is sharing as different from the transfer keyword in ionic compouunds. Since the electron is shared, it is controlled by both atom since it is still within their valence shells
A practical application can be seen in the case of carbon (iv) oxide
Here, we have a central carbon atom and two side oxygen atoms
The carbon atom has the shell configuration of 2,4 (it needs 4 extra electrons to achieve stability).
For the oxygen , the configuration is 2,6 (they both need 2 each since they are 2)
So, there must be an interaction.
Hence, we can have the 4 from the carbon shared 2 ,2 . But it will be within the two shells.
The carbon has 8 now ( 4 itself and 2 each from the contributing oxygen)
For the oxygen, they have 8 too (6 itself and 4 distributed 2, 2 for each oxygen)
This type of covalent bonding is the ordinary covalent bonding.
An example of this compound is carbon (iv) oxide
Covalent bonding occurs between atoms of similar electronegativity such as between non metals.
The difference between the two is that in ionic bonding, there is transfer of electrons while in covalent bonding, there is sharing of electrons
How many grams would 8.88 x 1029 atoms of NaCl be equivalent to?
Answers
Answer:
8.65x10 7 grams of nacl
Explanation:
There are 6.02x10 23 atoms per one mol
so
8.88x 10 29 x (1/6.02 x 10 23)
Will give us mols of NaCl
to go from mols to grams we multiple mols by molar mass
so
1.48x10 6 mols of Nacl x 58.44 g/mol = 8.65x10 7 grams of nacl which can be converted to
86500 kg
how do I solve this organic chemistry question?

Answers
Answer:
a) 3,3-dimethyl-2-butanol
b) Attached below
c) Yes, this compound is optically active because it has one chiral carbon. Highlighted as Red below.
d) Structures attached below.
e) Isomers shown below.

A certain compound is 66.7% carbon, 3.74% hydrogen, and 29.6% oxygen. Find the empirical formula.
Answers
Answer: C3H2O
Explanation: To find the empirical formula of the compound, we need to determine the simplest whole number ratio of atoms in the compound.
Assuming we have 100 g of the compound, we can convert the percentages to masses of each element:
Carbon: 66.7 g
Hydrogen: 3.74 g
Oxygen: 29.6 g
Next, we need to convert these masses to moles using the atomic masses:
Carbon: 66.7 g / 12.01 g/mol = 5.55 mol
Hydrogen: 3.74 g / 1.01 g/mol = 3.70 mol
Oxygen: 29.6 g / 16.00 g/mol = 1.85 mol
Now we need to divide each of these mole values by the smallest of the three:
Carbon: 5.55 mol / 1.85 mol = 3.00
Hydrogen: 3.70 mol / 1.85 mol = 2.00
Oxygen: 1.85 mol / 1.85 mol = 1.00
These ratios give us the empirical formula:
C3H2O
However, we can simplify this formula by dividing each subscript by 2:
C1.5H1O0.5
Finally, we can multiply through by 2 to get rid of the decimals:
C3H2O
what is the maximum amount of KNO3 that can be dissolved in 100 g of 50 C water ? A. 15g B. 36g C. 84g D. 100g

Answers
From the solubility curve, it is clear that 84 g of potassium nitrate can be dissolved in 100 g of water at 50°C.
What is solubility?Solubility is defined as the ability of a substance which is basically solute to form a solution with another substance. There is an extent to which a substance is soluble in a particular solvent. This is generally measured as the concentration of a solute present in a saturated solution.
The solubility mainly depends on the composition of solute and solvent ,its pH and presence of other dissolved substance. It is also dependent on temperature and pressure which is maintained.Concept of solubility is not valid for chemical reactions which are irreversible. The dependency of solubility on various factors is due to interactions between the particles, molecule or ions.
Learn more about solubility,here:
https://brainly.com/question/8591226
#SPJ1
Calculate the solubility of MgF2 in water if the Ksp for the compound is 6.4 x 10-9.
Answers
The solubility of MgF₂ in water is 0.00635 moles per liter, if Ksp for the compound is 6.4 x 10⁻⁹.
The solubility of a compound in water is determined by its solubility product constant (Ksp), which is a measure of the extent to which the compound dissociates into its constituent ions in water.
For the given compound, magnesium fluoride (MgF₂), the Ksp is 6.4 x 10⁻⁹.
MgF₂ dissociates in water according to the following equation;
MgF₂(s) ↔ Mg²⁺(aq) + 2F⁻(aq)
The Ksp expression for MgF₂ is then;
Ksp = [Mg²⁺] × [F⁻]²
where [Mg²⁺] represents the concentration of Mg²⁺ ions in solution, and [F⁻] represents the concentration of F⁻ ions in solution.
Since MgF₂ dissociates into one Mg²⁺ ion and two F⁻ ions, the stoichiometry of the reaction is 1:2. This means that for every mole of MgF₂ that dissolves, one mole of Mg²⁺ ions and two moles of F⁻ ions are formed.
Let's assume that the solubility of MgF₂ in water is "x" moles per liter. Therefore, the concentration of Mg²⁺ ions and F⁻ ions in solution will also be "x" moles per liter.
Substituting these values into the Ksp expression, we get;
Ksp = [Mg²⁺] × [F⁻]²
6.4 x 10⁻⁹ = x × (2x)²
6.4 x 10⁻⁹ = 4x³
Now, we can solve for "x";
4x³ = 6.4 x 10⁻⁹
x³ = (6.4 x 10⁻⁹ / 4
x³ = 1.6 x 10⁻⁹
x = (1.6 x 10⁻⁹(1/3)
x ≈ 0.00635
Therefore, the solubility of MgF₂ in water is approximately 0.00635 moles per liter.
To know more about solubility here
https://brainly.com/question/22185953
#SPJ1
ion
Р
Question 6
1321 ✪
9 words
Consider the reaction 3X + 2Y→ 5C + 4D
How many moles of C can be synthesized from 33.0 moles of Y?
Round your answer to a whole number.
1 pts
Answers
Answer:
83
Explanation:
3X + 2Y → 5C + 4D
2 moles of Y will produce 5 moles of C
33.0 moles of Y will produce: 33.0 x 5/2 = 82.5 or 83 moles of C
Question 9Valence electronsAHave low energy and are found in the outermost enery levelBHave the highest energy and found in the outermost energy levelСHave the highest energy and found in the closet energy levelDHave low energy and are found in the closet energy level
Answers
Answer:
\(B\)Explanation:
Here, we want to select the option true of valence electrons
Valence electrons are electrons that reside in the outermost shell of the atom
They have the highest energy of the different kinds of electrons present in the atom
Due to their very high energy levels, they reside in the farthest position from the nucleus which is the outermost or valence shell
The correct answer here is thus B
What law says the total amount of
substance at the beginning of a
reaction must match the amount of
substance at the end of the reaction?
A. Law of Conservation of Energy
B. Law of Conservation of Moles
C. Law of Conservation of Heat
D. Law of Conservation of Mass
Answers
Answer:
d) law of conservation of mass
Explanation:
__________ are composed of two or more covalently bonded atoms.
a). elements
b)molecules
Answers
look at the reaction below and state which direction the reaction would shift:
2Hg0 <=> 2Hg +O2
Answers
Answer:
there is no shift in the state
Explanation:
The correct answer is - There is no shift in the state.
Reason -
If K > Q, a reaction will proceed forward, converting reactants into products. If K < Q, the reaction will proceed in the reverse direction, converting products into reactants. If Q = K then the system is already at equilibrium.
where Q, is the reaction Quotient
What is the wavelength of a yellow light with a frequency of 5.2 x 10¹⁴ Hz?
[?] × 10[?] m
c = 3.0 x 10^8 m/s
Answers
the wavelength of a yellow light with a frequency of 5.2 x 10^14 Hz is approximately 5.77 x 10^-7 meters.
How to calculate wavelength?The speed of light, c = 3.0 x 10^8 m/s, is a constant in the universe. The wavelength of light, λ, and its frequency, f, are related to the speed of light by the formula:
c = λ × f
Rearranging the formula to solve for the wavelength, we get:
λ = c / f
Substituting the given values, we get:
λ = (3.0 x 10^8 m/s) / (5.2 x 10^14 Hz)
= 5.77 x 10^-7 m
Therefore, the wavelength of a yellow light with a frequency of 5.2 x 10^14 Hz is approximately 5.77 x 10^-7 meters.
Learn about wavelength here https://brainly.com/question/10728818
#SPJ1
The AH° of the reaction is -1367 kJ. Calculate the work done on the system at 25°C. C2H5OH(1) + 302(g) → 2CO2(g) + 3H₂O(0)
Answers
The work done on the system for the reaction \(C2H5OH(1) + 3O2(g) → 2CO2(g) + 3H2O(0)\) at 25°C is 1240 kJ.
The work done on a system can be calculated using the Gibbs free energy equation: \(\beta ΔG = ΔH - TΔS,\) where ΔH is the enthalpy change, T is the temperature, and ΔS is the entropy change. The given enthalpy change is -1367 kJ, which represents the amount of heat released during the reaction.
To calculate the work done on the system at 25°C, we need to calculate the entropy change.
\(ΔS°\)(C₂H₅OH) = 160.7 J/K·mol
ΔS°(O₂) = 205.0 J/K·mol
ΔS°(CO₂) = 213.8 J/K·mol
ΔS°(H₂O) = 188.8 J/K·mol
Using the formula \(ΔS° = ΣnS°(products) - ΣmS°(reactants)\), we can calculate the entropy change for the reaction. This gives:
\(ΔS° = [2(213.8 J/K·mol) + 3(188.8 J/K·mol)] - [1(160.7 J/K·mol) + 1(205.0 J/K·mol) + 3(188.8 J/K·mol)]\)
\(ΔS° = -470.9 J/K·mol\)
Now we can calculate the work done on the system using the Gibbs free energy equation:
\(ΔG = ΔH - TΔS\)
\(ΔG = -1367 kJ - (25°C + 273.15)K × (-0.4709 kJ/K·mol)\)
\(ΔG = -1240 kJ/mol\)
The negative sign indicates that the work is done on the system, and its magnitude is 1240 kJ. Therefore, the work done on the system for the reaction \(C2H5OH(1) + 3O2(g) → 2CO2(g) + 3H2O(0)\) at 25°C is 1240 kJ.
To learn more about Gibbs free energy here
https://brainly.com/question/13318988
#SPJ1
If your equation includes 7(CrO4)2, how many Cr's are there?
If your equation includes 7(CrO4)2, how many O's are there?
Answers
If an equation includes 7(CrO₄)₂, the numbers of Cr's and O's atoms that are there are 14 and 56 respectively.
How to calculate number of atoms?The number of atoms present in a chemical compound can be calculated by multiplying the subscript of the particular element by any coefficient.
According to this question, 7 moles of chromate with the chemical formula; (CrO₄)₂ is given. The number of oxygen and chromium atoms in this compound can be calculated as follows:
Chromium = 7 (coefficient) × 2 = 14 atomsOxygen = 7 (coefficient) × 8 = 56 atomsLearn more about no of atoms at: https://brainly.com/question/14190064
#SPJ1
130 cm of a gas at 20°C exerts a pressure of
750 mm Hg. Calculate its pressure if its volume
is increased to 150 cm3 at 35 °C.
Answers
Answer: The pressure is 1137.5 mm Hg its pressure if its volume is increased to 150 \(cm^{3}\) at 35 °C
Explanation:
Given: \(P_{1}\) = 750 mm Hg, \(V_{1} = 130 cm^{3}\), \(T_{1} = 20^{o}C\)
\(P_{2}\) = ?, \(V_{2} = 150 cm^{3}\), \(T_{2} = 35^{o}C\)
Formula used to calculate the new pressure is as follows.
\(\frac{P_{1}V_{1}}{T_{1}} = \frac{P_{2}V_{2}}{T_{2}}\)
Substitute the values into above formula as follows.
\(\frac{P_{1}V_{1}}{T_{1}} = \frac{P_{2}V_{2}}{T_{2}}\\\frac{750 mm Hg \times 130 cm^{3}}{20^{o}C} = \frac{P_{2} \times 150 cm^{3}}{35^{o}C}\\P_{2} = 1137.5 mm Hg\)
Thus, we can conclude that the pressure is 1137.5 mm Hg its pressure if its volume is increased to 150 \(cm^{3}\) at 35 °C.
The periodic table shows that a carbon atom has six protons. This means
that a carbon atom also has
*
O Six electrons
An atomic mass that equals six
O Six neutrons
O
More protons than electrons
Answers
Answer:
A carbon atom has six protons. This means that a carbon atom also has
a) Six electrons
b) Six neutrons
Explanation:
A wooden block has a mass of 10 g with volume of 20 mL what is the density of the block
Answers
Answer:
100
Explanation:
Equal chemical equations!
H2 + Cl2 ----> HCl
P4 + O2 ----> P4O10
Na + Cl2 ----> NaCl
CH4 + O2 ----> CO2 + H2O
Answers
Answer:Na+Cl2---->NaCl
Explanation:
Answer:
I've balanced the equations.
They are in the attached image.
The values in red are the added number to make the equation balanced .

Which is an example of health technology?
A. Television
B. Vaccines
C. Light bulbs
D. Swimming pools
Answers
Answer:
B
Explanation:
Vaccines prevent illness and disease
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8