A sample of 3.62 moles of diphosphorous trioxide is
reacted with 6.31 moles of water to produce
phosphorous acid. Using the balanced equation
below, predict which is the limiting reactant and the
maximum amount in moles of phosphorous acid that
can be produced.
P2O3 + 3H₂O → 2H3PO3
A. water, 8.42 moles
B. water, 4.21 moles
C. diphosphorus trioxide, 7.22 moles
D. diphosphorus trioxide, 3.75 moles
Answers
\(H_3PO_3\)\(2H_3PO_3\)\(2H_3PO_3\)\(P_2O_3\)Answer:
B
Explanation:
This question is about stoichiometry. From the balanced equation \(P_2O_3 + 3H_2O\)⇒\(2H_3PO_3\), we see that 3 moles of water is needed to react with 1 mole of \(P_2O_3\).
This means that, to fully react 3.62 moles of \(P_2O_3\), we would need 3*3.62 or 10.86 moles of water. However, we only have 6.31 moles, so water is the limiting reactant.
Since 3 moles of water react with 1 mole of \(P_2O_3\), 6.31 moles of water can fully react with 6.31÷3 or 2.1033 moles of \(P_2O_3\).
From the balanced equation, we see that every mole of \(P_2O_3\) reacted gets you 2 moles of \(2H_3PO_3\). Therefore, 2.1033 moles of \(P_2O_3\) would give you approximately 4.21 moles of \(H_3PO_3\).
Related Questions
Thermal energy always moves from a greater energy level to a lesser energy level
Theory or law?
Give an explanation please
Answers
Answer:
\(\fbox{Law -Based \: on \: laws \: of \: thermodynamic}\)
Explanation:
At constant pressure Thermal energy always moves from a greater energy level to a lesser energy level, laws of thermodynamics prove that.
Nature always likes to attain equilibrium either it's movement of heat energy or flow of water from higher region to lower region. The first and second law of thermodynamics are profe of that, the first law says that the total energy of universe is Constant. Energy can not be destroyed it always changes from one form to another, by work and heat. The second law explains why thermal energy moves from a greater energy level to a lesser energy level, it deals with the change in entropy of a system and surrounding and states heat flows from hot environment to cold environment.
Thanks for joining brainly community!
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
A solution has a [H3O+] of 1 × 10−5 M. What is the [OH−] of the solution?
A) 9 M
B) 14 M
C) 1 x 10^{-9}
D) 1 x 10^{-14}
Answers
Based on the kinetic theory, which statement is true? (5 points)
O Matter consists of only large molecules.
Matter is made up of only charged particles.
The particles of matter have zero kinetic energy and potential energy.
O The particles of matter are arranged in different ways for the different states.
Answers
Answer:
the particles of matter are arranged in different ways for the different states
Explanation:
because solid liquid and gas all three matters have different states for example the particles in a solid are closely packEd and form of movement is vibration
Someone help thank you :)

Answers
draw the structural formula(s) for the organic product(s) formed when the following compound is heated at reflux in an excess of concentrated .
Answers
1,3 diiodo propane is formed when oxetane is heated at reflux.
Reflux is defined as the process which involves heating the chemical reaction for a specific amount of time, while continually cooling the vapor produced back into liquid form, using a condenser. The vapors that are produced above the reaction continually undergo condensation, returning to the flask as a condensate.
Oxetane is a stable carbon compound which shows characteristic properties when reacted with conc. acids. When oxetane reacts with HI it gets reduced to form 1,3 diiodo propane which is also an organic compound.
Therefore, when oxetane is heated in excess amount of concentrated HI, the product formed is 1,3 diiodo propane.
The reaction is given in the image attached below.
The given question is incomplete and the completed question is given below.
Draw the structural formula(s) for the organic product(s) formed when the following compound (OXETANE) is heated at reflux in an excess of concentrated (HI) .
Learn more about reflux from the link given below.
https://brainly.com/question/29484768
#SPJ4

An object has a mass of 3.40 oz and a volume of 0.1159 L . What is the density ( g/mL ) of the object?
Answers
According to the question, the density of the object is 0.831 g/mL.
What is Density?Density may be defined as a type of physical property that significantly involves the measurement of quantity or mass per unit of volume in a particular substance.
Density is further characterized as the ratio of mass per unit volume. The unit of density can be expressed in g/cm³ or kg/m³.
According to the question, the mass of an object is = 3.40 oz.
Convert ounce into grams:
1 ounce = 28.35 grams.
3.40 ounces = 3 × 28.35 = 96.38 g.
The volume of an object = 0.1159 L. = 115.9 mL.
Now, the formula for calculating density = \(\frac{mass}{volume}\)
= 96.38/115.9 = 0.831 g/mL.
Therefore, the density of the object is 0.831 g/mL.
To learn more about Density, refer to the link:
https://brainly.com/question/6838128
#SPJ1
Trees often lose their leaves in the winter. Explain what this tree will not be able to do without its leaves.
Answers
Answer:
Most aren't able to take in carbon dioxide gas from the air or produce any oxygen.
Explanation:
science bro
To identify a diatomic gas ( X2 ), a researcher carried out the following experiment: She weighed an empty 6.4- L bulb, then filled it with the gas at 1.30 atm and 27.0 ∘C and weighed it again. The difference in mass was 9.5 g . Identify the gas.
Answers
The diatomic gas ( X2 ) is N₂ dinitrogen.
Dinitrogen is a chemical compound fashioned from the covalent bonding of two nitrogen atoms. it's far a colorless, odorless gas at room temperature and pressure, which makes up about seventy-eight % of the Earth's environment.
Diatomic gas is a chemical compound formed from the covalent bonding of two nitrogen atoms. it's miles drab, odorless gasoline at room temperature and stress, which makes up about seventy eight % of the Earth's surroundings.
Volume = 6.4 L
Pressure = 1.3 atm
Temperature = 25 C = 298 K
R = 0.08206 L.atm/mol.K
P * V = n * R * T
1.3 atm * 6.4 L = n * 0.08206 L.atm/mol.K * 298 K
n = 0.34 moles
difference of mass is the mass of gas = 9.5 g
Molar mass = Mass / No. of moles = 9.5 g / 0.34 moles = 27.9 g/mol
diatomic gas with molar mass 28 g/mol is N2
Hence the diatomic molecule is N2
Learn more about dinitrogen here:-https://brainly.com/question/11651796
#SPJ9
Calculate the volume of O2, at STP, required for the complete combustion of 125g octane (C8H18) to CO2 and H20
Answers
306.178 liters is the volume of O2 at STP, required for the complete combustion of 125g octane (\(C_{8}H_{18}\)) to \(CO_{2}\) and H20
To calculate the volume of \(O_{2}\) required for the complete combustion of octane (\(C_{8}H_{18}\)) to \(CO_{2}\) and \(H_{2}O\) at STP (Standard Temperature and Pressure), we need to consider the stoichiometry of the balanced chemical equation.
The balanced equation for the combustion of octane is:
\(C_{8}H_{18}\) + 12.5\(O_{2}\) -> 8\(CO_{2}\) + 9\(H_{2}O\)
From the equation, we can see that 1 mole of octane requires 12.5 moles of \(O_{2}\) to completely combust. The molar mass of octane (\(C_{3}H_{18}\)) is approximately 114.22 g/mol.
To calculate the moles of octane, we divide the given mass by the molar mass:
Moles of octane = 125 g / 114.22 g/mol ≈ 1.093 mol
Since the molar ratio between octane and \(O_{2}\) is 1:12.5, the moles of \(O_{2}\)required can be calculated as:
Moles of \(O_{2}\) = 1.093 mol * 12.5 ≈ 13.663 mol
Now, we can use the ideal gas law, PV = nRT, to calculate the volume of \(O_{2}\) at STP. At STP, the temperature is 273 K, and the pressure is 1 atm.
Using the molar volume of an ideal gas at STP (22.4 L/mol), the volume of \(O_{2}\) required is:
Volume of \(O_{2}\) = Moles of \(O_{2}\) * Molar volume = 13.663 mol * 22.4 L/mol ≈ 306.178 L
Therefore, approximately 306.178 liters of \(O_{2}\) at STP would be required for the complete combustion of 125 grams of octane (\(C_{8}H_{18}\)) to \(CO_{2}\) and \(H_{2}O\)
Know more about combustion here:
https://brainly.com/question/10458605
#SPJ8
Student was identifying the formula of 1 mole of unknown powder. When it was placed on a scale, it was reading 399.91 g/mol. Which of
the following compounds can it be?
A AI(NO3)3
B. C3H8
C. Fe2(SO4)3
D. CaCl2
Answers
Answer: The compound is \(Fe_2(SO_4)_3\)
Explanation:
Molar mass is defined as the mass in grams of 1 mole of a substance.
S.I Unit of Molar mass is gram per mole and it is represented as g/mol.
A. 1 mole of \(Al(NO_3)_3\) weighs = 26.98(1)+14.01(3)+15.99(9) =212.99 g/mol
B. 1 mole of \(C_3H_8\) weighs = 12.01(3)+1.007(8) = 44.1 g/mol
C. 1 mole of \(Fe_2(SO_4)_3\) weighs = 55.84(2)+32.06(3)+15.99(12) = 399.91 g/mol
D. 1 mole of \(CaCl_2\) weighs = 40.07(1)+35.5(2)= 110.98 g/mol
Thus the compound is \(Fe_2(SO_4)_3\)
What is the molar absorptivity of the cu2+ at 780 nm, given that the cuvette had a path length l of 1 cm?.
Answers
It is not stated what the molar absorptivity of Cu2+ at 780 nm with a 1 cm route length is. Depending on the particular solution and circumstances, the molar absorptivity value changes.
What does molar absorptivity mean?A species' molar absorptivity determines how much of a specific wavelength of radiation it can absorb. A species is stimulated from its ground state to a more energetic excited state as electromagnetic radiation is absorbed.
In Beer's law, what does molar absorptivity mean?What about the variable (often called a) in the Beer's law equation? The sample's molar absorptivity, commonly referred to as the extinction coefficient, is expressed as. It is a special physical constant of the sample's chemistry that has to do with its capacity to absorb light of a particular wavelength.
To know more about molar absorptivity visit:
https://brainly.com/question/4862658
#SPJ4
how many moles are in 6.7 x 10^25 molecules of H2SO4
Answers
Answer:
\( \huge{ \boxed{111.30 \: \: \text{moles}}}\)
Explanation:
To find the number of moles in a substance given it's number of entities we use the formula
\( \bold{n = \frac{N}{L} \\ }\)
where
n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities.
From the question.
N = 6.7 × 10²⁵ \( \: H_2SO_4 \: \) molecules
\(n = \frac{6.7 \times {10}^{25} }{6.02 \times {10}^{23} } \\ = 111.2956...\)
We have the final answer as.
111.30 molesWhen the rate of the forward reaction equals the rate of the backward reaction, the system is said to be in (2 points)
a. reverse
b. dynamic equilibrium
c. homeostasis
d. suspended state
Answers
Answer:
I believe b, dynamic equilibrium
Explanation:
What you know about rolling ' down in the deep?
When your brain goes numb, you can call that mental freeze
When these people talk too much, put that sh ei t in slow motion, yeah
I feel like an astronaut in the ocean, aye
What you know about rolling' down in the deep?
When your brain goes numb, you can call that mental freeze
When these people talk too much, put that s h e it in slow motion, yeah
I feel like an astronaut in the ocean
Answers
Answer:
She say that im cool
I'm like "yeah thats true"
I believe in G-O-D
Dont believe in T-H-O-T
Explanation:
Answer:
rolling in the deep
Explanation:




Rank each structural feature according to increasing energy difference between the ground state and excited state electrons. (lowest on top)
Answers
The structural feature according to increasing energy difference between the ground state and excited state electrons is given as (i) conjugated polyene (ii) conjugated diene (iii) isolated diene.
Generally, chemical energy is defined as the energy which is stored in the bonds of chemical compounds (molecules and atoms). Chemical energy is released in the chemical reaction and mostly produces heat as a by-product which is known as an exothermic reaction.
The ground state of an electron is defined as the energy level that it usually occupies, is the state of lowest energy for that electron. There is also a maximum energy present that each electron can have and still be part of its atom.
Generally, the excited state describes an atom, ion or molecule with an electron in a higher than normal energy level than its ground state.
Learn more about energy difference from the link given below.
https://brainly.com/question/14681215
#SPJ4
What type of particle has no mass or charge?
A) Alpha
B) Beta
C) Gamma
D) Proton
Answers
Answer:
Your answer would be C.
Explanation:
Gamma radiation, unlike alpha or beta, does not consist of any particles, instead consisting of a photon of energy being emitted from an unstable nucleus. Having no mass or charge, gamma radiation can travel much farther through air than alpha or beta, losing (on average) half its energy for every 500 feet.
Gamma particles has no mass or charge.
What are gamma radiations?Gamma radiations is a kind of radiations, which are emitted during a nuclear reaction. Gamma radiations are emitted in the form of photons of energy, they did not carry any particles like alpha, beta and proton. So, because of not carrying any particle this radiations have no mass and as it is on the form of energy they also do not carry any charge.
Hence, the particles which has no mass or charge is gamma.
To learn more about gamma radiations, visit the below link:https://brainly.com/question/20799041
Hail is falling down from the sky at 50 miles per hour. Is this an example of speed,
velocity or acceleration?
Answers
Explanation:
tgggghhhhijyyhhjjjjjjjj
What do scientists mean when they refer to population
Answers
population is the number of species living in a given area if this is regarding biology or earth studies :)
Pewter is a solidified solution of tin and lead or tin and zinc. In both cases, tin is the main component. Which metal would you classify as the solute in each type of pewter?
Answers
__ N2 + __ H2 → __ NH3
A. How many moles of ammonia (NH3) can be produced if 0.75 moles of N2 are reacted with enough hydrogen gas?
B. Extension Problem: How many moles of H2 gas are needed to react with 56.0 g of N2? Hint: Remember you can only apply the mole ratio on moles, not grams, but what can you do with grams first?
Answers
A total of 1.5 moles of ammonia (NH₃) could be produced.
To react with 56.0 g of N₂, 6.000 moles of H₂ gas are required.
A. We need to balance the chemical equation first:
N₂ + 3H₂ → 2NH₃
From the balanced equation, we can see that 1 mole of N₂ reacts with 3 moles of H₂ to produce 2 moles of NH₃.
Therefore, if 0.75 moles of N₂ are reacted with enough hydrogen gas, we can calculate the moles of NH₃ produced using the mole ratio:
0.75 moles N₂ × (2 moles NH₃ / 1 mole N₂) = 1.5 moles NH₃
Therefore, 1.5 moles of NH₃ can be produced.
B. We can use the molar mass of N₂ to convert 56.0 g of N₂ to moles:
56.0 g N₂ × (1 mole N₂ / 28.02 g) = 2.000 mole N₂
From the balanced equation, we know that 1 mole of N₂ reacts with 3 moles of H₂ to produce 2 moles of NH₃.
Therefore, the moles of H₂ required can be calculated using the mole ratio:
2.000 mole N₂ × (3 mole H₂ / 1 mole N₂) = 6.000 mole H₂
Therefore, 6.000 moles of H₂ are needed to react with 56.0 g of N₂.
To know more about the Ammonia, here
https://brainly.com/question/30873620
#SPJ1
Which of the following is NOT an advantage of a wireless network? convenience reliability security speed
Answers
Answer:
Security
Explanation:
A wireless network consists of good connection with fast speed, convenience, and reliability. Security is a major problem with wireless connection as someone can get your information by joining the same connection as you.
Will give Brainliest!
A student titrates 25.0 mL of an unknown base with 0.10 M HCl. During the titration the pH is monitored and the collected data is recorded. These data are shown in the table below.
Volume
Added(mL) pH
0.0 11.13
5.0 9.86
10.0 9.44
12.5 9.26
15.0 9.08
20.0 8.66
22.0 8.39
24.0 7.88
25.0 5.28
26.0 2.70
28.0 2.22
30.0 2.00
35.0 1.70
37.5 1.61
40.0 1.52
45.0 1.40
50.0 1.30
a. Use the information provided to draw a titration curve showing the pH as a function of the volume of added HCl. Be certain to label your axes.
b. Identify the equivalence point on your graph and justify your selection of this particular point.
b. Use the data to determine the Kb value for the weak base. Be certain to show the mathematical steps you take to arrive at the answer. Report your final answer to the correct number of significant digits.
c. The student has three indicators that she could use for this experiment. The indicators (with their endpoints) are: Bromophenol Blue (3.0 – 4.6), Methyl Red (4.2 – 6.3), and phenolphthalein (8.3 – 10.0). Which indicator would be appropriate for this titration? Justify your selection.
e. Determine the (i) molarity and the (ii) % ionization of the original weak base solution (before titrating). Report your answers to the correct number of significant digits.
Answers
a. Titration Curve:
On the x-axis, label it as "Volume of HCl added (mL)"
On the y-axis, label it as "pH"
b. Equivalence Point:
The equivalence point is the point in the titration where the moles of acid (HCl) added are stoichiometrically equivalent to the moles of base (unknown base) present initially. In the given data, the equivalence point can be estimated to be around 25.0 mL of HCl added. This is where the pH drops dramatically from 7.88 to 5.28, indicating the neutralization of the base.
c. Calculation of Kb Value:
To determine the Kb value, we need to find the pOH at half-neutralization, where half the volume of the equivalent point has been reached. In this case, the half-neutralization volume is 12.5 mL (half of 25 mL).
From the data, we can observe that at 12.5 mL of HCl added, the pH is 9.26.
pOH = 14 - pH = 14 - 9.26 = 4.74
pOH = -log[OH-]
[OH-] = 10^(-pOH)
[OH-] = 10^(-4.74)
To find [OH-] in moles per liter (M), we need to convert mL to L.
[OH-] = 10^(-4.74) mol/L
Now, since we know that at the equivalence point, the concentration of the acid (HCl) is 0.10 M, we can use the stoichiometry of the reaction to determine the concentration of the base (unknown base).
From the balanced equation:
HCl + OH- → H2O + Cl-
1 mole of HCl reacts with 1 mole of OH-
0.10 M (HCl) = [OH-] M (unknown base)
Therefore, Kb = [OH-][unknown base] / [base]
Kb = (10^(-4.74) mol/L)(0.10 M) / (0.10 M - 10^(-4.74) M)
Simplify and calculate Kb.
c. Selection of Indicator:
Based on the given pKa ranges of the indicators, the indicator phenolphthalein (pKa range: 8.3 - 10.0) would be appropriate for this titration. The reason is that the pH at the equivalence point is expected to be around 7, which is well within the range of phenolphthalein's color change. Bromophenol Blue and Methyl Red have lower pKa values and would not be suitable for indicating the equivalence point in this particular titration.
d. Calculation of Molarity and % Ionization of the Weak Base Solution:
To calculate the molarity of the weak base solution, we can use the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
At the half-neutralization point, [A-] = [HA], and the pH is 9.26.
9.26 = pKa + log([A-]/[HA])
The pKa can be determined using the pOH at half-neutralization:
pKa = 14 - pOH = 14 - 4.74 = 9.26
9.26 = 9.26 + log([A-]/[HA])
log([A-]/[HA]) = 0
[A-]/[HA] = 10^0 = 1
Since [A-] = [HA], the concentration of the weak base (before titration) is equal to the concentration of its conjugate acid.
Therefore, the molarity of the weak base solution is 0.10 M.
To calculate the % ionization of the weak base, we can use the formula:
% Ionization = ([A-]/[HA]) × 100
% Ionization = (1/0.10) × 100
% Ionization = 1000%
Note: The % ionization may exceed 100% in cases where the concentration of the conjugate acid is very small compared to the concentration of the weak base.
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
Based on a Kc value of 0.250 and the given data table, what are the equilibrium concentrations of XY, X, and Y , respectively?
Answers
From the solution that we have in the question;
The concentration of X and Y is 0.28 MThe concentration of XY is 0.32 MWhat is the equilibrium constant?The equilibrium constant, denoted as K, is a value that quantitatively represents the ratio of the concentrations of products to reactants at equilibrium in a chemical reaction.
It is a fundamental concept in chemical equilibrium.
The value of the equilibrium constant provides valuable information about the position of equilibrium and the relative concentrations of species involved in a chemical reaction.
Kc = [X] [Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\)
\(0.25(0.5 - x) = (0.1 + x)^2\)
\(0.125 - 0.25x =0.01 + 0.2x + x^2\\ x^2 + 0.45x - 0.115 = 0\)
x = 0.18 M
The equilibrium amount of X and Y= 0.28 M and the equilibrium concentration of XY = 0.32 M
Learn more about equilibrium constant:
https://brainly.com/question/29253884
#SPJ1
Based on the answer to the question that we have;
A 0.28 M concentration of X and Y exists at equilibriumXY's concentration at equilibrium is 0.32 M.The equilibrium constantThe ratio of the product to reactant concentrations in a chemical reaction at equilibrium is represented quantitatively by the equilibrium constant, abbreviated as K.
It is a cornerstone of the theory of chemical equilibrium.
A chemical reaction's equilibrium position and the relative concentrations of the species involved can both be learned from the equilibrium constant's value.
Kc = [X][Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\\0.25(0.5 - x) = (0.1 +x)^2\\0.125 - 0.25x = 0.01 +0.2x +x^2\\= 0.18 M\)
The equilibrium concentration of;
XY =0.5 - 0.18
=0.32 M
Then the equilibrium amount of
X and Y is
0.1 + 0.18= 0.28 M.
Learn more about equilibrium:brainly.com/question/29253884
#SPJ1

O Macmillan Learning
What is the IUPAC name for the compound shown?
IUPAC name:
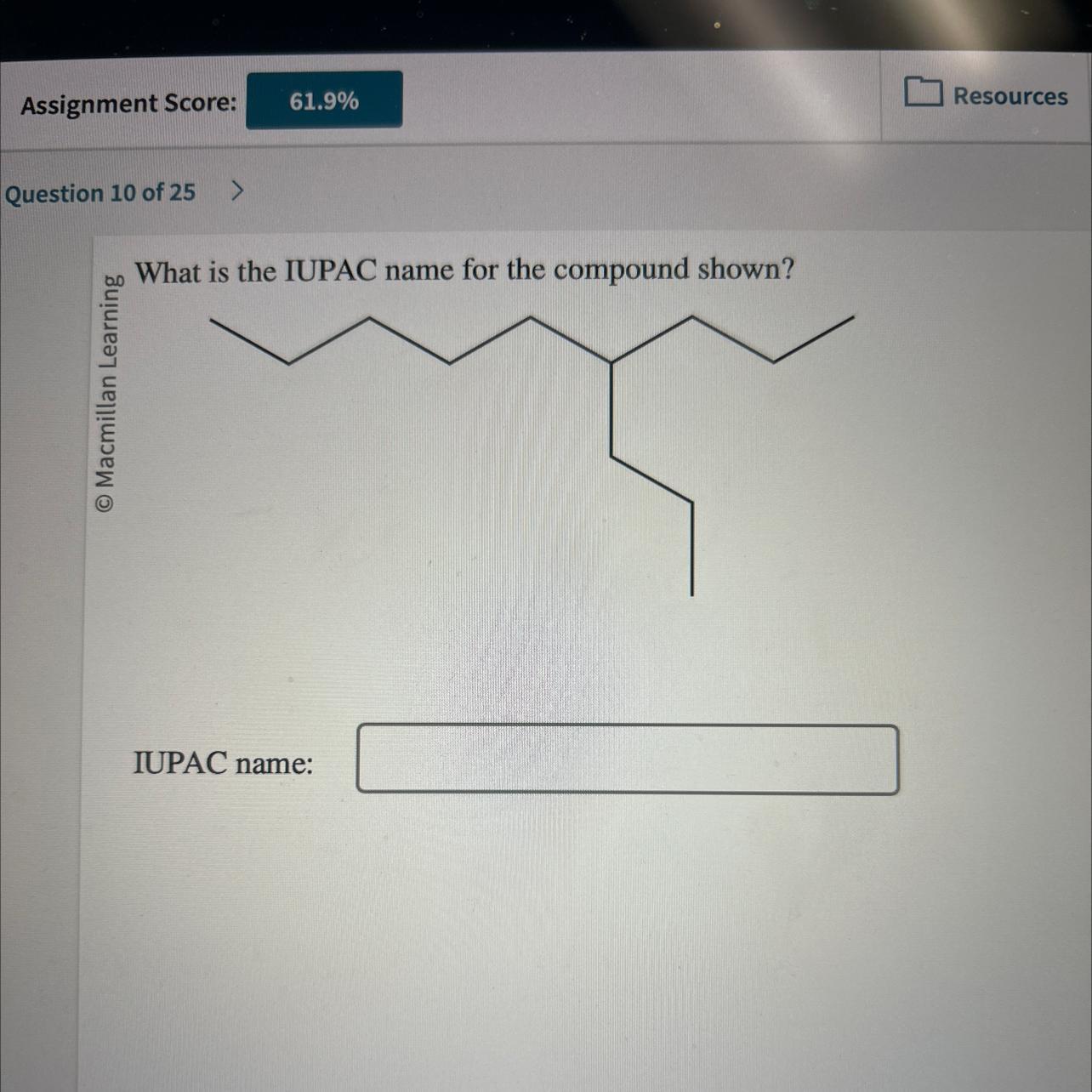
Answers
The compound shown has the IUPAC name O Macmillan Learning. -3-ethyl-2,2-dimethyl-hexane Incorrect.
Why was 1 6 dimethylhexane wrong?Explanation and response: Because it implies that there's methyl groups on carbons number one and sixth of the parent carbon chain, the name — appears-dimethylhexane is incorrect. Because the name "hexane" implies that a parent atom chain only has six molecules long, the methyl groups are located at the ends of every molecule.
Is hexane considered an organic chemical?Hexane, commonly referred to as sextane, is an organic compound that belongs to the alkane class. They are acyclic branched as well unbranched hydrocarbons with the standard structure CnH2n+2, and thus entirely composed of hydrogen and saturated oxygen atoms. Hexane is a colorless, clear liquid.
To know more about dimethylhexane visit :
https://brainly.com/question/30438544
#SPJ1
Convert 11.9 g of Be(NO2)2 to moles.
Answers
To convert grams (g) of a substance to moles (mol), you need to divide the mass by the molar mass of the substance.
The molar mass of Be(NO2)2 can be calculated by adding the molar masses of its constituent atoms, which are:
The molar mass of Be: 9.01 g/mol
The molar mass of N: 14.01 g/mol
The molar mass of O: 16.00 g/mol (there are two oxygen atoms in the nitrite ion, NO2-)
So the molar mass of Be(NO2)2 is:
9.01 g/mol (Be) + 2(14.01 g/mol (N) + 2(16.00 g/mol (O)) = 9.01 g/mol + 28.02 g/mol + 32.00 g/mol = 69.03 g/mol
Now we can use this molar mass to convert 11.9 g of Be(NO2)2 to moles:
moles = mass/molar mass
moles = 11.9 g / 69.03 g/mol
moles ≈ 0.1727 mol
Therefore, 11.9 g of Be(NO2)2 is equivalent to approximately 0.1727 moles.
Magine that you have a metal bar sitting half in the Sun and half in the dark. On a sunny day, the part of the metal that has been sitting in the Sun feels hot. If you touch the part of the metal bar that has been sitting in the dark, will it feel hot or cold?
Answers
Answer:
The metal will feel hot.
Explanation:
Metals are known for their high thermal conductivity. If one part of the metal feels hot, the other will as well.
_______________________________________________________
This piece of metal has been exposed to the sun, and will feel hot after a certain amount of time. By the statement above, the unexposed part will feel hot as well.
Hope that helps!
What was one idea Dalton taught about atoms?
A. Atoms contained negatively charged particles scattered inside.
B. Atoms of one type would not react with atoms of another type.
C. All atoms of one type were identical in mass and properties.
D. Atoms changed into new elements when they formed compounds.
Answers
Answer:
C
Explanation:
I had this question and C is the right answer
One idea that Dalton taught about atoms was that all atoms of one type were identical in mass and properties.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ5
3 H2SO4(aq) + 2 Al(s) → Al2(SO4)3(aq) + 3 H2(g) How many moles of Al2(SO4)3 are produced when 6.5 moles of aluminum are consumed?
Answers
Answer:
3.25 moles
Explanation:
From the equation, 2 moles of Al results in one mole of Al2(so4)3
6.5 / 2 = 3.25