a photon with energy 2.92 ev is absorbed by a hydrogen atom. (a) find the minimum n for a hydrogen atom that can be ionized by such a photon. 3 correct: your answer is correct. (b) find the speed of the electron released from the state in part (a) when it is far from the nucleus. incorrect: your answer is incorrect.
Answers
The electron will be released from the atom and become ionized if a photon has greater energy than the electron's binding energy.
How much photon energy does a hydrogen atom require to ionize before it leaves its ground state?E1=13.6 eV is the minimal amount of energy needed to break free of the ground state. E 1 = 13.6 e V . Here, E stands for the overall energy needed to ionize an atom of hydrogen.
What energy level does a photon need to be at in order to ionize a hydrogen atom from the n 3 energy level?The formula En=2.181018n2 J/atom denotes the minimal amount of energy required to ionize an electron from a hydrogen atom's nth Bohr orbit in Joules per atom.
To know more about photon visit:-
https://brainly.com/question/20912241
#SPJ4
Related Questions
PLEASE QUICKLY. I'll give BRAINLIEST. A sample contains 25% parent isotope and 75% daughter isotopes. If the half-life of the parent isotope is 72 years, how old is the sample?
144 years old
216 years old
288 years old
360 years old
Answers
From the calculations and the principles of radioactivity, the age of the parent isotope is 144 years
What is radioactivity?The term radioactivity has to do with the spontaneous decay of a substance.
We know that we have about 25% parent isotope still remaining hence;
\(0.693/t1/2 =2.303/t log No/N\\When N =0.25 No and t1/2 = 72 years\\0.693/72 =2.303/t log No/0.25No\\0.0096 = 2.303/t *0.60206\\0.0096 = 1.3865/t\\t = 1.3865/0.0096\\t =144 years\)
Learn more about radioisotope:https://brainly.com/question/13076859
#SPJ2
Answer:
A. 144 years old
Explanation:
Calculate the density of an object with a mass of 15.6 grams and a volume of 2 cm3
Answers
Answer:
7.8 g/cm³
Explanation:
The density of an object can be defined as the object's mass divided by its volume:
Density = mass / volumeWe are given both the mass and volume by the problem, so we compute the data in the equation:
Density = 15.6 g / 2 cm³Density = 7.8 g/cm³This means the density of the object is 7.8 g/cm³.
gallium (III) carbonate formula
Answers
Can someone please give me a clear definition of what ceramics are? It will really help! :)
Answers
The pots and other articles made from clay hardened by heat is called ceramics..
HOPE IT'S HELPFUL FOR U MATE!!!!
a stone is projected horizontally with 20 metre per second from the top of a tall building calculate its position in velocity after 3 seconds neglecting the air resistance .
Answers
Answer:
105m
Explanation:
u=20m/s
g=10m/s^2
t=3s
s=ut+1/2gt^2at
s=20*3+1/2*10*3*3
s=105m
Explain how evidence of a glacier appearing in India supports continental drift.
Answers
Using the phase diagram for H₂O, what phase is water in at 1 atm pressure
and -5°C?
A. It is at its melting point.
B. It is in the gas phase.
C. It is in the liquid phase.
D. It is in the solid phase.
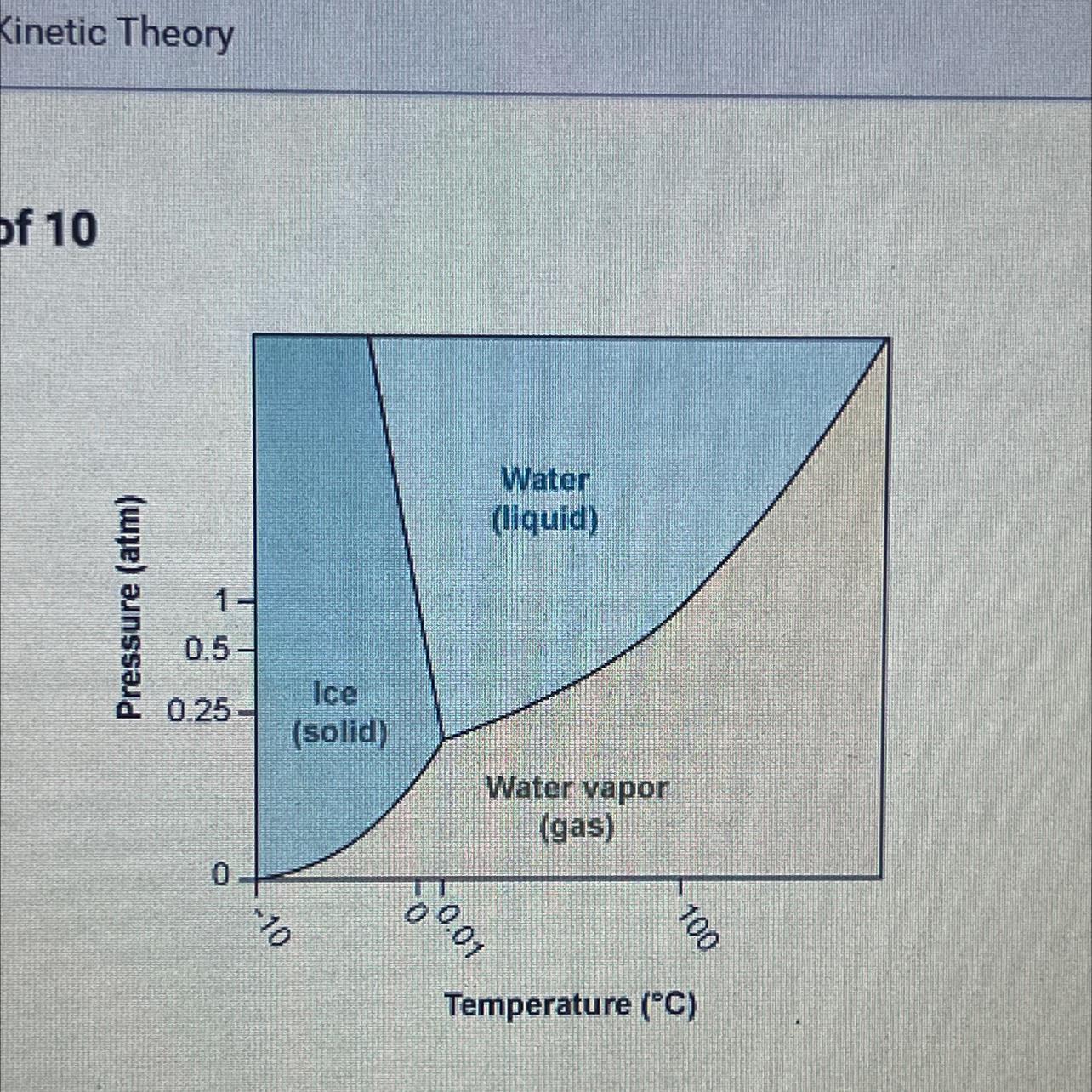
Answers
The number of phases that exist in equilibrium in a system depends upon the variables like temperature, pressure and composition. Here at 1 atm pressure water is in solid phase. The correct option is D.
What is phase diagram?The phase equilibria studies are made simpler by the use of plots which show how the various equilibria depend on the temperature, pressure and composition variables. These diagrams are called phase diagrams.
Here the pressure 1 atm on y-axis coincides with the temperature -5°C on x-axis. The water system is a one component system. So the water is in solid phase.
Thus the correct option is D.
To know more about phase diagrams, visit;
https://brainly.com/question/16945664
#SPJ9
I need help finding the answer to this question

Answers
Answer:
1.1 for 3d, 0.76 for 16d.
Explanation:
You divide the mass of one nail by the nail size.... I think
To deploy configuration profiles for computers from Jamf Pro, _____ must be available.
a) Global Service Exchange
b) Apple Business Manager
c) Apple School Manager
d) Apple Push Notification service
Answers
To deploy configuration profiles for computers from Jamf Pro, the Apple Push Notification service (APNs) must be available.
APNs is a cloud-based service provided by Apple that enables the secure transfer of data between Apple devices and servers. It is essential for communication between Jamf Pro and Apple devices during the deployment of configuration profiles.
APNs is used to initiate the connection between the devices and Jamf Pro, allowing for the transfer of the configuration profiles. Without APNs, it would be impossible to deploy configuration profiles to Apple devices using Jamf Pro.
Therefore, it is critical to ensure that APNs is available and functioning correctly before attempting to deploy configuration profiles using Jamf Pro.
To learn more about : Apple
https://brainly.com/question/13148332
#SPJ11
Give a brief description of the symptoms associated with dissociative fugue.
Answers
Answer:
People may seem and act normally during the fugue, or they may appear moderately bewildered and draw no notice. When the fugue is over, however, people are thrown into a new scenario with no recall of how they got there or what they were doing.
Explanation:
9. What is the process of nuclear fusion? *
(10 Points)
O A. The process of carrying light
B. The process of two atoms fusing
O C. The process of gravity and inertia keeping the universe together
O D. The process of fusing two stars
Answers
Answer:
the process of carrying light
Hydrogen gas and oxygen gas are produced when sodium sulfate solution is electrolysed. Explain how oxygen gas is produced in the electrolysis of sodium sulfate solution. [4 marks
Answers
When electricity is passed through sodium sulfate solution, it separates the water molecules. Oxygen gas is produced at one electrode called the anode. At the other electrode called the cathode, hydrogen gas is produced. So, during electrolysis, oxygen gas is made at the anode and hydrogen gas is made at the cathode.
psoriasis is a disease that causes a red rash on the skin. which body system does psoriasis affect?
a. respiratory
b. integumentary
c. immune
d. digestive
e. circulatory
Answers
Answer:
The answer to this question is integumentary.
Explanation:
The answer is that because that is your skin area and so if you have a rash then if will affect that part.
It's b. integumentary
I got the test right
If you know about crystals can you tell me if this is real or fake because I purchased this item and don’t know

Answers
carbon dioxide and particulates are emitted by volcanoes. particulates form stratospheric aerosols that reflect sunlight. which of the following best describes the impact of atmospheric carbon dioxide and stratospheric aerosols?
Answers
Carbon dioxide and particulates are emitted by volcanoes. Particulates form stratospheric aerosols that reflect sunlight The impact of atmospheric carbon dioxide and stratospheric aerosols can be briefly described below.
Carbon dioxide (CO₂): Carbon dioxide is a greenhouse gas that contributes to the greenhouse effect and global warming. It is released into the atmosphere through various human activities, such as the burning of fossil fuels and deforestation. The increasing concentration of CO₂ in the atmosphere is a significant driver of climate change.
Stratospheric aerosols: Stratospheric aerosols, formed by the release of particulates from volcanic eruptions or human activities, can have a cooling effect on the Earth's climate. These aerosols reflect sunlight back into space, reducing the amount of solar radiation that reaches the Earth's surface. As a result, they can temporarily offset some of the warming caused by greenhouse gases like CO2.
It's important to note that while stratospheric aerosols can have a cooling effect, they are relatively short-lived in the atmosphere compared to CO₂. Carbon dioxide, on the other hand, has a long residence time and accumulates over time, leading to long-term warming.
Therefore, the overall impact of increasing atmospheric CO₂ concentrations outweighs the cooling effect of stratospheric aerosols.
Learn more about stratospheric aerosols here:
https://brainly.com/question/28073358
#SPj 4
Answer if you know the answer //Which of the following is an unusual property of water?
A. Its atoms are bound by ionic bonds.
B. It doesn't exist in the gaseous state on Earth.
C. Its charge is balanced evenly around the atom.
D. It's a polar molecule.
Answers
H2O is covalent so A is false.
Water is in clouds so B is false.
Water is polar so C is false.
D is correct H2O has an imbalance of e- so it is polar. \(+H-O(-)-H+\)
An unusual property of water is a polar molecule. Therefore, option D is correct.
What is polar molecule ?When two atoms form a covalent bond but do not share their electrons equally, polar molecules are created. A dipole is created when a portion of the molecule has a slight positive charge and a slight negative charge.
Because the electrons of the hydrogen atoms are "drawn" toward the electrons of the oxygen atom, water (H2O) is a polar molecule. As a result, the hydrogen atoms have a positive charge, whereas the opposite end of the molecule has a negative charge.
Polarity is one of the special characteristics of water because it is composed of polar molecules. Each water molecule has one oxygen atom and two hydrogen atoms (H2O). Polar covalent bonds are created by these compounds. The atoms of hydrogen are positively charged.
Thus, option D is correct.
To learn more about the polar molecule, follow the link;
https://brainly.com/question/24775418
#SPJ5
pls help me 13 min left if you go past and not help are not mine

Answers
Answer:
mushroom
mold
spore cases
hyphae
fungus
the term used to denote concentration of electrolytes in a given volume is
Answers
The term used to denote the concentration of electrolytes in a given volume is "molarity".
Molarity-
Molarity is the number of moles of solute dissolved in one liter of the solution. Molarity is the most widely used concentration metric in chemistry, and it is frequently denoted by "M." It denotes the amount of solute in moles per liter of solution.
A solution's molarity can be calculated using the following formula:
Molarity = moles of solute / liters of solution
Molarity is often used to express the concentration of a solution's electrolytes because electrolytes break into ions when dissolved in a solvent, allowing for electrical conductivity.
Molarity can be used in other applications, such as stoichiometry, which involves determining how much of one compound is required to react completely with another.
Molarity is a useful tool for solving problems that involve chemical reactions since the number of moles of a substance is frequently used to establish reaction ratios, limit reactants, and determine the theoretical yield.
Learn more about the molarity from the given link-
https://brainly.com/question/30404105
#SPJ11
Which of these accurately describes a chemical reaction?
A. Products and reactants combine to form new reactants
B. Products and reactants combine to form new substances
C. Products combine to make reactants
D. Recatants combine to form products
Answers
Answer:
D
Explanation:
its just right
Answer:Reactants combine to form Products. Hope this helped! ;)
Explanation:
¿How do the products of the reaction to the phenol red test and the splint test? Please help me it's for today.! :((
Answers
Answer:
The answer in this question is show you made Sodium Hydroxide and Hydrogen Gas.In order to do the products of the reaction relate to the phenol red test and the splint test you need to show that you made Sodium Hydroxide and Hydrogen Gas. Show that you made Sodium Hydroxide and Hydrogen Gas so that the products of the reaction relate to the phenol red test and the splint test.
In balancing the nuclear reaction 238/92U → 234/90E + 4/2He, the identity of element E is ________
Answers
The identity of element E in the nuclear reaction 238/92U → 234/90E + 4/2He is thorium (Th). The main ans for balancing nuclear reactions is to ensure that the total mass and atomic numbers are conserved on both sides of the reaction.
In this case, the total mass on the left side (238) is equal to the sum of the masses on the right side (234 + 4). Similarly, the total atomic number on the left side (92) is equal to the sum of the atomic numbers on the right side (90 + 2). Therefore, the only element that satisfies these conditions and has an atomic number of 90 is thorium (Th). This explanation confirms that the identity of element E in the nuclear reaction is thorium (Th).
In the nuclear reaction 238/92U → 234/90E + 4/2He, the identity of element E is Thorium (Th). To find the identity of element E, you need to look at the atomic number provided (90). Element 90 on the periodic table is Thorium (Th). In this reaction, Uranium (238/92U) decays into Thorium (234/90Th) and an alpha particle (4/2He).
To know more about thorium visit :
https://brainly.com/question/14156154
#SPJ11
A student develops their TLC plate and places it under a UV light, but nothing appears. What mistake might the student have made?
Answers
A possible mistake the student might have made is not using a proper solvent system for their sample on the TLC plate. If the sample components are too polar or nonpolar compared to the solvent, they might not move effectively on the plate, making it difficult to observe under UV light.
Additionally, the student could have applied too little sample, causing the spots to be too faint to see under UV light. It is important for the student to optimize the solvent system and sample application to achieve proper separation and visualization of components on the TLC plate under UV light. The wavelength of UV light, also known as ultraviolet light, is shorter than that of visible light but longer than that of X-rays. According to its wavelength, UV light may be classified as UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (200–280 nm). The least dangerous UV-A radiation causes tanning and skin ageing. Sunburn is brought on by UV-B, which, when exposed in excess, can also result in skin cancer.
Learn more about UV light here:
https://brainly.com/question/25724408
#SPJ11
How many grams of carbon dioxide are produced when 16 g of methane and 48 g of oxygen gas combust
Answers
Need help ASAP please

Answers
Answer:Melting can create steam, kind of like a nukeular plant exept no nukulear rods
2. Exothermic; decreases
3. Endothermic;increases
4. Exothermic; decreases
5. Endothermic; increases
3. Complete the following conversions.
a. 10.5g=__kg
b. 1.57 km = ___m
c. 3.54 ug = ___g
d. 3.5 mol=___umol
e. 1.2 L = ___mL
f. 358 cm = ____m3
g. 548.6 ml. =____cmii
Answers
Answer:
a. 0.0105
b. 1.570
c. 0.00000354
d. 35,000,000
e. 1200
f. 0.000358
g. 548.6
The complete conversion of the given examples are as follows:
a. 10.5g = 0.00105 kg
b. 1.57 km = 1570 m
c. 3.54 ug = 3.54 × 10⁻⁵ g
d. 3.5 mol = 3.5 × 10⁶ umol
e. 1.2 L = 1200 mL
f. 358 cm = 3.58 × 10⁻⁴ m³
g. 548.6 ml = 548.6 cm
Conversion is a multi-stage procedure, which requires a numerical factor to multiply or divide and the appropriate selection of a number of important digits.
Using the International System Units for Conversions Factors.
(a)
If 1 g = 0.001 kg
∴
10.5 g will be:
\(\mathsf{= \dfrac{10.5 \ g \times 0.0001 \ kg}{1 \ g}}\)
= 0.00105 kg
(b)
If 1 km = 1000 m
1.57 km will be:
\(\mathsf{= \dfrac{1.57 \ km \times 1000\ m}{1 \ km} }\)
= 1570 m
(c)
If 1 ug = 1 × 10⁻⁵ g
∴
3.54 ug will be:
\(\mathsf{= \dfrac{3.54 \mu g \times 1 \times 10^{-5} \ g }{1 \ \mu g}}\)
= 3.54 × 10⁻⁵ g
(d)
If 1 mol = 10⁶ umol
∴
3.5 mol will be:
\(\mathsf{= \dfrac{3.5 \ mol \times 10^6 \ u \ mol}{1 \ mol} }\)
= 3.5 × 10⁶ umol
(e)
If 1 L = 1000 mL
∴
1.2 L will be:
\(\mathsf{=\dfrac{1.2 \ L \times 1000 \ mL}{1 \ L}}}\)
= 1200 mL
(f)
If 1 cm = 0.000001 m³
∴
358 cm will be:
\(\mathsf{= \dfrac{358\ cm \times 0.000001 m^3}{1 \ cm}}\)
= 3.58 × 10⁻⁴ m³
(g)
If 1 mL = 1 cm
∴
548.6 mL will also be equivalent to 548.6 cm
Therefore, from the above explanation, we've seen how to convert from one unit to another.
Learn more about conversion rates here:
https://brainly.com/question/5685229?referrer=searchResults
Calculate the volume of ethyl alcohol that evaporated (in mL). 1 L = 1,000 mL and 1 mole of ANY gas at STP = 22.4 L.
Answers
To calculate the volume of ethyl alcohol that evaporated, we need to know the volume of ethyl alcohol that was present initially, the volume of ethyl alcohol after the evaporation, and the molar mass of ethyl alcohol.
Let's assume that the initial volume of ethyl alcohol is V_0 mL and the final volume is V_f mL. The volume of ethyl alcohol that evaporated is then V_e = V_0 - V_f mL.
We can use the ideal gas law to relate the volume of a gas to the number of moles of the gas present. The ideal gas law is given by:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature.
Since the volume of a gas is directly proportional to the number of moles of the gas, we can write:
V/n = k
where k is a proportionality constant.
If we know the value of k for a given gas at a particular temperature and pressure, we can use this relationship to calculate the number of moles of the gas present.
In this case, we are told that 1 mole of any gas at STP (standard temperature and pressure) occupies a volume of 22.4 L, which is equivalent to 22,400 mL. Therefore, the value of k for any gas at STP is 22,400 mL/mol.
We can use this value of k to calculate the number of moles of ethyl alcohol that evaporated:
n = V_e/(22,400 mL/mol)
Substituting in the known values for V_e and k, we can calculate the number of moles of ethyl alcohol that evaporated.
For example, if the initial volume of ethyl alcohol was 1,000 mL and the final volume was 900 mL, the volume of ethyl alcohol that evaporated would be V_e = 1,000 mL - 900 mL = 100 mL. The number of moles of ethyl alcohol that evaporated would be:
n = 100 mL/(22,400 mL/mol) = 0.0044 moles
I hope this helps! Let me know if you have any questions.
what atom is weakly electronegative and would be more stable if it gave up a valence electron?
Answers
Potassium atom is weakly electronegative and would be more stable if it gave up a valence electron.
The propensity of an element to draw a pair of bonding electrons is measured by its electronegativity. The Pauling measure is most frequently employed. The number ranges from 4.0 for fluorine, the most electronegative element, to 0.7 for cesium and francium, the least electronegative elements.
What happens if two elements with equivalent electronegativity combine?
Think about the connection between elements A and B. Since both atoms have the same propensity to attract the bonding pair of electrons if they are equally electronegative, it will typically be located halfway between the two atoms.
Normally, A and B would have to be the same atom to form a connection like this. This kind of bond can be found in compounds like H2 or Cl2, for instance. It's essential to keep in mind that this is a typical image. Actually, the electrons are in a molecule orbital, where they are constantly revolving. The electrons in this type of connection are distributed equally between the two atoms, making it a "pure" covalent bond.
To know more about electronegative:
https://brainly.com/question/17762711
#SPJ4
What is the speed of a cheetah that runs 30 miles in 0.5 hours?
a
Answers
Answer:
60 mph (miles per hour)
Explanation:
0.5 hours is 1/2 of an hour, so to get the number of miles for a whole hour you multiply the miles ran by 2.
30 times 2 is 60.
S = 30/0.5 = 60mi/ hr
3. SE7.12D The cell organelle responsible for providing energy for cell processes in plant
and animal cells is the -
Answers
The osmotic pressure of a solution containing 15. 87 mg of an unknown protein per 10. 0 mL of solution is 2. 45 torr at 25oC. Find the molar mass of the unknown protein
Answers
After calculation and analyzing the molar mass of the unknown protein is
103.92g/mol.
the formula for the molar mass is
Π = i x M x R x T
here,
Π = osmotic pressure
i = van't Hoff factor
M = molarity
R = constant
T = temperature
therefore, staging the given values into the formula we get
2.45 = 1 x M x 62.3637
M = 0.000104mol/L
now the molar mass of the protein is
molar mass = mass/ moles
given
15.87 gm of protein in 10ml
then,
0.01587 g of protein in 10 ml
restructuring the formula concerning moles
moles = mass/molar mass
0.01587 / 0.000104
= 0.153 mol/L
hence, the molar mass of the unknown protein is
molar mass = 0.01587/0.153
molar mass = 103.92 g/mol
After calculation and analyzing the molar mass of the unknown protein is
103.92g/mol.
To learn more about molar mass,
https://brainly.com/question/837939
#SPJ4