A person inhales air richer in O₂ and exhales air richer in CO₂ and water vapor. During each hour of sleep, a person exhales a total of about 300 L of this CO₂-enriched and H₂O-enriched air.
(b) How many grams of body mass does the person lose in 8 h of sleep if all the CO₂ and H₂O exhaled come from the metabolism of glucose? C₆H₁₂O₆(s) + 6O₂(g) → 6CO₂(g) + 6H₂O(g)
Answers
Answer:
It would take like aleast 200,000 kilogram of body mass because the total is aleast 300L
Explanation:
Related Questions
What is the ph of h3o+ = 2.4(10^-3) m?
a.) 3
b.) 2.6
c.) 5.4
d.) -3
Answers
Answer:
b). 2.6The pH of a solution can be calculated using the formula: pH = -log[H+], where [H+] is the concentration of hydrogen ions in the solution. In this case, we are given the concentration of hydronium ions (H3O+), which is the same as the concentration of hydrogen ions in an aqueous solution. So, [H+] = 2.4(10^-3) m Taking the negative log of this value, we get: pH = -log(2.4(10^-3)) pH = 2.62
About hydrogenHydrogen or water as it is sometimes called, is a chemical element on the periodic table that has the symbol H and atomic number 1. At standard temperature and pressure, hydrogen is a colorless, odorless, non-metallic, single-valent, and highly diatomic gas flammable. Hydrogen can be used as an energy source, energy storage, energy carrier, to be used for infrastructure purposes.
You can learn more about Hydrogen at https://brainly.com/question/24433860
#SPJ11
What is the coefficient for water molecules in the balanced version of the following redox reaction? cr2o2−7 c2h4o→c2h4o2 cr3
Answers
The given redox reaction is:
Cr2O7^2- + C2H4O → C2H4O2 + Cr3+
To balance this reaction, we first balance the oxygen atoms by adding H2O on the right side of the equation. The number of H2O molecules added depends on the number of oxygen atoms needed. In this case, we need three O atoms on the right side, so we add three H2O molecules to the right side of the equation:
Cr2O7^2- + C2H4O → C2H4O2 + Cr3+ + 3H2O
Next, we balance the hydrogen atoms by adding H+ ions on the left side of the equation. The number of H+ ions added depends on the number of hydrogen atoms needed. In this case, we need eight H atoms on the left side, so we add eight H+ ions to the left side of the equation:
Cr2O7^2- + C2H4O + 8H+ → C2H4O2 + Cr3+ + 3H2O
Finally, we balance the charge by adding electrons. The number of electrons added depends on the difference in charge on the left and right side of the equation. In this case, the left side has a charge of -2 (from the Cr2O7^2- ion), while the right side has a charge of +3 (from the Cr3+ ion). This means that we need to add 5 electrons to the left side of the equation to balance the charge:
Cr2O7^2- + C2H4O + 8H+ + 5e- → C2H4O2 + Cr3+ + 3H2O
Therefore, the coefficient for water molecules in the balanced version of the given redox reaction is 3.
To know more about redox reaction visit
https://brainly.com/question/28300253?
#SPJ11
According to the electronegativity difference between the atoms in water, it would be appropriate to label hydrogen with the symbol 18+ and oxygen with the symbol 8+ st
Answers
The electronegativity of hydrogen is 2.0, while that of oxygen is 3.5.
Electronegativity is a chemical property that describes an atom's or functional group's tendency to attract electrons toward itself. An atom's electronegativity is affected by both its atomic number and the distance between its valence electrons and the charged nuclei.
It governs the distribution of shared electrons between two atoms in a bond. The greater an atom's electronegativity, the more strongly it attracts electrons in its bonds. The electronegativity of hydrogen is 2.0, while that of oxygen is 3.5. Because the difference in electronegativities is 1.5, water is a polar covalent molecule. This means that electrons are strongly attracted to the more electronegative element, but the atoms are not ionized.
To learn more about electronegativity, here
https://brainly.com/question/17762711
#SPJ4
How to know if a single replacement reaction will occur
Answers
Answer:
To determine whether a given single replacement will occur, you must use an “Activity Series” table. If the metal or the halogen is above the element it will replace based on the activity series, a single displacement reaction will occur. The video below summarizes an experiment conducted to compare the activities of three metals ( Mg, Cu and Zn ).
Explanation:
Single replacement reaction will occur if any more active specie will add on the solution.
What is single displacement reaction?Single displacement reactions are those reactions in which one element from the given molecule is displaced by some other element.
Generally displacement reactions are shown by metals when more reactive metal is added in the solution of any another metal. Then more reactive metal displaces the metal which is already present in the solution and reaction is taking place is shown by changing in color.
Hence single displacement reaction will occur when more reactive metal is added in any solution.
To know more about single displacemen reaction, visit the below link:
https://brainly.com/question/13328989
#SPJ4
You must evaluate the purchase of a proposed spectrometer for the R&D department. The base price is $140,000, and it would cost another $30,000 to modify the equipment for special use by the firm. The equipment falls into the MACRS 3-year class and would be sold after 3 years for $60,000. The applicable depreciation rates are 33%, 45%,15%, and 7%, as discussed in Appendix 12 A. The equipment would require an $8,000 increase in net operating working capital (spare parts inventory). The project would have no effect on reventies, but it should save the firm $50,000 per year in before-tax labor costs. The firm's marginal federal-plus-state tax rate is 35%. a. What is the initial investment outlay for the spectrometer, that is, what is the Year 0 project cash flow? b. What are the project's annual cash flows in Years 1, 2, and 3 ? c. If the WACC is 9%, should the spectrometer be purchased? Explain.
Answers
a. To determine the initial investment outlay for the spectrometer, we need to calculate the cash flows at Year 0. The base price of the spectrometer is $140,000, and the modification cost is $30,000.
Thus, the initial cost is the sum of these two amounts, which is
$170,000 ($140,000 + $30,000).
Additionally, we need to consider the increase in net operating working capital, which is $8,000. Therefore, the initial investment outlay for the spectrometer is
$178,000 ($170,000 + $8,000).
b. In Years 1, 2, and 3, we need to calculate the annual cash flows. Firstly, we consider the savings in before-tax labor costs, which is $50,000 per year. Next, we calculate the depreciation expense using the MACRS depreciation rates provided. In Year 1, the depreciation expense is
$140,000 * 0.33 = $46,200. In Year 2,
it is $140,000 * 0.45 = $63,000.
In Year 3, it is $140,000 * 0.15 = $21,000.
Finally, we subtract the depreciation expense from the before-tax labor cost savings to get the annual cash flows. In Year 1, it is
$50,000 - $46,200 = $3,800. In Year 2,
it is $50,000 - $63,000 = -$13,000. In Year 3,
it is $50,000 - $21,000 = $29,000.
c. To determine whether the spectrometer should be purchased, we need to calculate the net present value (NPV) of the project's cash flows. Using the WACC of 9%, we discount the cash flows in each year. The NPV is the sum of the discounted cash flows. If the NPV is positive, the project is considered favorable.
If it is negative, the project should not be pursued. In this case, we calculate the NPV by discounting the cash flows in Years 1, 2, and 3 at a rate of 9%. The NPV is calculated as: NPV = ($3,800 / (1 + 0.09)^1) + (-$13,000 / (1 + 0.09)^2) + ($29,000 / (1 + 0.09)^3). If the NPV is positive, the spectrometer should be purchased; if it is negative, it should not be purchased.
To know more about working visit:
.https://brainly.com/question/14921083
#SPJ11
20.00 cm³ of a solution containing 0.53g 14 of anhydrous Na2CO3 in 100cm³ requires is 25.00cm³ of H₂SO4 for complete neutralization. The concentration of the acid solution in moles per dm³ is A. 0.02 cm³ B. 0.04cm³ C. 0.06cm³ p D. 0.08 cm³ [H= 1, C = 12, O= 16, Na= 23, S =32]
Answers
The concentration of the H2SO4 solution is 1.00 mol/dm³ or 0.08 cm³/mol. Option D.
Stoichiometric problemThe balanced chemical equation for the reaction between Na2CO3 and H2SO4 is:
Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2
From the equation, we can see that 1 mole of Na2CO3 reacts with 1 mole of H2SO4. Therefore, the number of moles of H2SO4 used in the reaction is:
moles of H2SO4 = 25.00/1000 × C
where C is the concentration of the H2SO4 solution in mol/dm³.
The number of moles of Na2CO3 in the solution is:
moles of Na2CO3 = mass of Na2CO3 / molar mass of Na2CO3moles of Na2CO3 = 0.53 / (2 × 23 + 12 + 3 × 16)moles of Na2CO3 = 0.005 molSince 20.00 cm³ of the solution contains the above amount of Na2CO3, then 100 cm³ of the solution contains:
moles of Na2CO3 in 100 cm³ = (0.005 mol / 20.00 cm³) × 100.00 cm³moles of Na2CO3 in 100 cm³ = 0.025 molSince the reaction between Na2CO3 and H2SO4 is a neutralization reaction, the number of moles of H2SO4 used in the reaction is equal to the number of moles of Na2CO3 in the solution. Therefore:
0.025 mol H2SO4 = 0.005 mol Na2CO3
Substituting the expression for moles of H2SO4 above, we get:
0.025 = (25.00/1000) × CC = 1.00 mol/dm³Therefore, the concentration of the H2SO4 solution is 1.00 mol/dm³ or 0.08 cm³/mol.
More on stoichiometric calculations can be found here: https://brainly.com/question/27287858
#SPJ1
Carry out the following calculation and express the answer with the appropriate number of significant figures: (2.4056 x 1.4002) + 0.0953 Do not enter units or spaces in your response. Carry out the following calculation and express the answer with the appropriate number of significant figures:
(3.292 x 0.0045) – 2.8951 Do not enter units or spaces in your response.
Answers
The answers for the calculations are 3.4633 and -2.880, both expressed with the appropriate number of significant figures.
For the first calculation: (2.4056 x 1.4002) + 0.0953 = 3.36800872 + 0.0953
Since the least number of significant figures in the given numbers is 4, the answer should have 4 significant figures: 3.368 + 0.0953 = 3.4633.
For the second calculation: (3.292 x 0.0045) - 2.8951 = 0.014814 - 2.8951
Since the least number of significant figures in the given numbers is 3, the answer should have 3 significant figures: 0.0148 - 2.895 = -2.880.
In each calculation, we perform the operations and consider the least number of significant figures in the given numbers to determine the appropriate number of significant figures in the final answer.
Summary:
The answers for the calculations are 3.4633 and -2.880, both expressed with the appropriate number of significant figures.
Learn more about significant figures click here:
https://brainly.com/question/24491627
#SPJ11
more nh3 gas is added to the system at time t2 while the temperature is held constant. which of the following will most likely occur? responses the value of the equilibrium constant will increase the value of the equilibrium constant will increase the value of the equilibrium constant will decrease. the value of the equilibrium constant will decrease. the total pressure in the container will decrease. the total pressure in the container will decrease. the amount of n2 will increase. the amount of n 2 will increase. the amount of h2 will decrease.
Answers
When more NH₃ gas is added to the system at time t₂ while the temperature is held constant, the value of the equilibrium constant will increase, the total pressure in the container will increase, the amount of N₂ will increase, and the amount of H₂ will decrease.
When more NH₃ gas is added to the system at time t₂ while the temperature is held constant, the equilibrium will shift to reduce the concentration of NH₃ and reach a new equilibrium state. According to Le Chatelier's principle, if the concentration of a reactant is increased, the equilibrium will shift to favor the products to balance the system.
Therefore, in this case, the equilibrium constant will shift towards the products, resulting in an increase in the value of the equilibrium constant. This is because the increase in NH₃ concentration will lead to an increase in the concentration of N₂ and H₂ as they are the products of the reaction.
However, the addition of more NH₃ gas to the system will also increase the total pressure in the container. This is because the number of gas molecules has increased, resulting in an increase in the overall pressure of the system. The amount of N₂ will increase due to the shift in equilibrium, while the amount of H₂ will decrease.
Learn more about equilibrium constants at https://brainly.com/question/3159758
#SPJ11
What happens if you overheat HNO₃?
Answers
HNO₃ decomposes on heating according to the equation:
4 HNO₃ ---> 2 H₂O + 4 NO₂ + O₂
What is HNO₃?HNO₃ is known as trioxonitrate (v) acid.
It is a volatile acid.
Like other acids, it turns blue litmus solution red and reacts with bases to form salt and water.
Being a volatile acid, when HNO₃ is overheated, it decomposes into several compound.
The equation for the reaction is given below:
4 HNO₃ ---> 2 H₂O + 4 NO₂ + O₂
In conclusion, HNO₃ decomposes on heating.
Learn more about trioxonitrate (v) acid at: https://brainly.com/question/22060503
#SPJ1
NEED IT ASAP!PLEASE HURRY

Answers
Answer:
snow, or freezing rain?
Explanation:
because freezing rain can occur then, but snow is more common.
Which of the following has the smallest atomic radius?
A. Astatine
B. Bromine
C. Fluorine
D. Iodine
Answers
Answer:fluorine
Explanation:
Explain how we know that charge is conserved in this
reaction: Li+ CI → Lici
Answers
Answer:
Charge is conserved due to the groups in which Lithium and Chlorine are located in the periodic table of the elements.
Explanation:
In the reaction \(Li + Cl - > LiCl\), we can examine the groups in which Li and Cl are found in the periodic table of the elements. Lithium appears in Group 1A, or the alkali metals group, indicating that it carries a charge of +1. Chlorine appears in Group 7A, or the halogen group, indicating that it carries a charge of -1. Because LiCl's constituent elements carry the same charges as previously mentioned, LiCl will have an overall charge of 0.
The chemical equation can then be rewritten as \(Li^{+} + Cl^{-} - > LiCl\), which, if looking at the individual charges of Li and Cl in lithium chloride, becomes \(Li^{+} + Cl^{-} - > Li^{+}Cl^{-}\). Adding the charges on the reactant and product sides of this chemical equation gives us zero in both locations, meaning that we have a charge of 0 on the reactant side and a charge of 0 on the product side. This indicates that charge is conserved in this reaction.
Another way to look at this is expressed in the valence electrons of Li and Cl. Li has an electron configuration of \(1s^{2}2s^{1}\), where the n = 2 electron shell has one of eight total electrons needed to fill the valence shell. This means that Li will easily lose one electron in order to have an electron configuration where the n = 1 electron shell is full, \(1s^{2}\), and become the \(Li^{+}\) ion. Similarly, Cl has an electron configuration of \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{5}\) (or \([Ne]3s^{2}3p^{5}\)), meaning that the n = 3 electron shell is one electron away from becoming complete. Cl will easily gain one electron to have the electron configuration \([Ne]3s^{2}3p^{6}\) (or \([Ar]\)) in order to have an electron configuration where the n = 3 electron shell is full, \(3s^{2}3p^{6}\), and become the \(Cl^{-}\) ion. Thus, when Li and Cl bond, Li will lose the electron \([1, 0, 0, +\frac{1}{2}]\) and transfer it to Cl, where it will become the electron \([3, 1, 1, -\frac{1}{2}]\), thus conserving charge, as there is an equal total number of electrons before and after the reaction.
1.4 Name three properties of discrete Fourier transform (DFT)
Answers
To summarize, the three properties of DFT include periodicity, time-shift, and linearity. The DFT is an important mathematical tool for signal processing, and it is utilized to transform a discrete-time sequence from the time domain to the frequency domain.
The three properties of Discrete Fourier Transform (DFT) are as follows:Periodicity: The discrete Fourier transform has a periodicity that is equal to the length of the data sequence. For example, if the DFT of a sequence of N points is performed, the resulting transform will repeat itself after N points of frequency or spectral information have been computed.Time-shift: The discrete Fourier transform is sensitive to the time shift of a sequence. For instance, the DFT of a time-shifted signal is a complex exponential multiplied by the DFT of the original sequence.Linearity: The discrete Fourier transform satisfies the principle of superposition. It implies that if two separate inputs x(n) and y(n) are given, then the transform of the sum of these two inputs is equal to the sum of the transform of the two inputs.To summarize, the three properties of DFT include periodicity, time-shift, and linearity. The DFT is an important mathematical tool for signal processing, and it is utilized to transform a discrete-time sequence from the time domain to the frequency domain.
To know more about processing visit:
https://brainly.com/question/31815033
#SPJ11
write a symbol equation showing the reaction of hydrogen with oxygen
Answers
Answer:
\(2H_{2(g)}+O_{2(g)}\text{ }\rightarrow2H_2O_{(l)}\)Explanation:
Here, we want to get the equation of reaction between hydrogen and oxygen
When hydrogen and oxygen react with the ration 2 to 1 in terms of number of moles, water is produced (2 moles of water)
We can show that as follows;
\(2H_{2(g)}+O_{2(g)}\text{ }\rightarrow2H_2O_{(l)}\)Which statement describes an electrolyte?
Answers
When an electrolyte dissolves in water, the resulting solution conducts an electric current.
If an object is accelerating the forces acting on the object are ?
Answers
Answer:
If an object is accelerating the forces acting on the object are BALANCED.
Explanation
if an object is moving at a constant rate of acceleration, the the forces acting upon it are balanced .
an acid and base react to form a salt and water in a(n) reaction. group of answer choices oxidation ionization neutralization reduction dissociation
Answers
An acid and a base react, to form salt and water in a neutralization reaction. The correct answer is neutralization.
Neutralization reaction is a chemical reaction that takes place when an acid and a base are mixed, resulting in the creation of a neutral solution, a salt, and water. The acid and base cancel each other out, producing a solution that is neither acidic nor basic.
An example of a neutralization reaction is the reaction between hydrochloric acid and sodium hydroxide, which creates salt and water as the only products.
The reaction can be represented as follows:
\(HCl\) + \(NaOH\) →\(NaCl\) + \(H_2O\)
An acid is a compound that contains hydrogen ions\((H^+)\) and can dissolve in water to produce a sour taste.
Bases are chemical substances that generate hydroxide ions \((OH^-)\) when dissolved in water.
Salt is a compound that can be formed by combining an acid and a base. Therefore, "neutralization" is the correct answer.
For more such questions on neutralization, click on:
https://brainly.com/question/23008798
#SPJ11
Find the ratio v/cv/c for an electron in the first excited state (n = 2) of hydrogen.
Answers
The answer is 0.365:100.
v/c ratio represents ratio of speed of an electron (v) to the speed of light (c).
How is the speed of an electron calculated?
The speed of an electron (v) is given by Bohr's model as-\(v =\frac{1}{n}\; \frac{e^{2}}{4\pi \varepsilon _{0}}\times \frac{2\pi }{h}\)
Now, for the first excited state, n = 2.
e - Charge of electron = \(1.6\)×\(10^{-19}\) C
h - Plank's constant = \(6.6\)×\(10^{-34} J.s\)
ε₀- permittivity \(= 8.85\)×\(10^{-12}\)\(N^{-1}.C^{2}.m^{-2}\)
Put the above data in the formula-\(v =\frac{1}{2}\; \frac{e^{2}}{4\pi \varepsilon _{0}}\times \frac{2\pi }{h} =\frac{1}{4}\times \frac{(1.6\times 10^{-19})^{2}}{8.85\times 10^{-12}\times 6.6\times 10^{-34}}\\ \\ =0.01096\times 10^{8} \\ \\=1.096\times 10^{6}ms^{-1}\)
Now, the speed of light, c = \(3.0\)×\(10^{8}\ m/s\)Thus, the v/c ratio for an electron in the first excited state is calculated as-\(\frac{v}{c} =\frac{1.096* 10^{6}\ m/s }{3.0 *10^{8}\ m/s }\)= \(\frac{0.365}{100}\)
Hence, the v/c ratio = 0.365:100.To learn more about the speed of an electron (v), visit:
https://brainly.com/question/13198566
#SPJ4
PLEASE HELP!!!!!!!!!!!!!!!!
What is a crosscutting concept for global temperature, explain.
Answers
Answer:
They include patterns; cause and effect; scale, proportion, and quantity; systems and system models; energy and matter; structure and function; and stability and change.
Explanation:
As such, they are a way of linking the different domains of science.
A dish is given to you, which contains a blackish yellow powder. When you move a magnet over it, black particles fly upwards and get stuck to the magnet. All that is left in the dish is a yellow powder, which you discover to be sulfur.
Was your original powder an element, compound, or mixture, and how do you know?
PLEASE ANSWER IF YOU HAVe an ANSWER OR NOt JUST FOR THE POINTS OR ELSE YOU WANT TO BE REPORTED!!!! please and thank you!
Answers
Answer:
It is a Mixture
Explanation:
It's a mixture because it was a substance made from mixing other substances together. And when the magnet went over the powder, the iron is lifted and not the sulfur. So the different parts of it can be separated by any physical means that allows the parts to seen separated
Answer:
its is a mixture
Explanation: because I did the question
Consider the molecule below. Determine the molecular geometry at each of the 2 labeled carbons. HELP ME PLEASE ASAP
C1 = trigonal planar, C2 = bent
B
C1 = trigonal planar, C2 = tetrahedral
C
C1 = bent, C2 = trigonal planar
D
C1 = tetrahedral, C2 = linear

Answers
The hybridization state of the atom determines its shape in the molecule. C1 is sp2 hybridized and has trigonal planar geometry while C2 is sp3 hybridized and has a tetrahedral geometry.
The shape of a molecule or a moiety in a molecule is determined by the number of electron pairs that are present on the valence shell of the central atom in the molecule.
The hybridization state of the atom also determines the shape of the atom. In the compound, the carbon atom labelled 1 is sp2 hybridized so it will have a trigonal planar geometry. The carbon atom labelled 2 is sp3 hybridized hence it is tetrahedral.
Learn more: https://brainly.com/question/10079361
What ions would the following elements form? Give the chemical symbol and the charge on the ion. [4]
a.Rubidium RbBr b. Bromine
b.Barium BaSO4 d. Sulfur
Answers
Answer:
N3-
Explanation:
A student is trying to identify an unknown mineral sample. The sample has a mass of 150 g and displaces 30 mL of water. Which mineral does the student have
please helpp
Answers
Which element will form an ionic bond with chlorine (Cl): bromine (Br) or magnesium (Mg)
Answers
Answer:
The answer is Magnesium
why, if we multiply a reaction by 2, don't we multiply its e°red by 2?
Answers
When we multiply a reaction by 2, we double the stoichiometric coefficients of the reactants and products.
However, the standard reduction potential (E°red) is an intensive property and remains unchanged. E°red represents the potential of a single mole of electrons transferred in the redox reaction. By doubling the reaction, we effectively double the number of moles of electrons transferred, but the potential per mole of electrons remains the same. Therefore, we do not multiply E°red by 2. It is important to note that E°red values are specific to individual half-reactions and do not depend on the overall balanced equation or the reaction stoichiometry.
Learn more about stoichiometric here:
https://brainly.com/question/6907332
#SPJ11
An Al-Si (aluminium-silicon) alloy is reinforced with Sumitomo alumina short fibre (diameter = 22 μm - micron) with the fibres aligned unidirectionally. Identify the most economical method to fabricate this composite – you need to justify your suggested fabrication route.
Answers
The most economical method to fabricate the Al-Si alloy reinforced with Sumitomo alumina short fibers would be the powder metallurgy route. This method involves mixing powdered aluminum and silicon with the alumina fibers, followed by compaction and sintering to create the composite material.
Powder metallurgy is a cost-effective fabrication method for composites due to several reasons. Firstly, it allows for efficient utilization of materials, as the powders can be precisely weighed and mixed in the desired proportions, minimizing waste. Secondly, the process can be easily scaled up for mass production, reducing production costs. Additionally, powder metallurgy offers good control over the distribution of reinforcement fibers, ensuring uniformity and enhancing the mechanical properties of the composite.
In the case of the Al-Si alloy reinforced with alumina fibers, the powder metallurgy route is advantageous because it enables the uniform dispersion of fibers throughout the matrix. The aligned unidirectional arrangement of fibers can be achieved by carefully controlling the mixing and compaction process. Furthermore, powder metallurgy allows for the incorporation of other additives or alloying elements to tailor the properties of the composite. Overall, the powder metallurgy route offers cost-effectiveness, process scalability, and control over fiber distribution, making it the most suitable and economical method for fabricating the Al-Si alloy reinforced with Sumitomo alumina short fibers.
To know more about Sumitomo alumina click here:
https://brainly.com/question/31592485
#SPJ11
How can the arrangement of furniture in a room influence an individual’s behavior?
a.
The arrangement of furniture in a public room can either encourage or discourage social interaction.
b.
The arrangement of furniture in a room can influence an individual’s attitude toward survival.
c.
An individual’s overall mental health can be severely negatively impacted by the arrangement of furniture in a room.
d.
An individual’s behavior cannot be influenced by the arrangement of furniture in a room.
Please select the best answer from the choices provided
Answers
The arrangement of furniture in a public room can either encourage or discourage social interaction. The correct option is A.
What is social interaction?It is the process of exchanging information with others, verbally or otherwise.
The arrangement of furniture in a room filled with people can go a long way to encourage or discourage social interactions.
If physical barriers are put in between two seats such that people can barely see each other, the message may be that very little to no social interaction is allowed.
Without any physical barriers between seats, the message may be that people are free to interact, all other things being equal.
More on social interactions can be found here: https://brainly.com/question/9648557
#SPJ1
Assume in a silicon sample Fermi level is 0.4eV below the intrinsic level E
1
. Find the carrier concentrations for both majority and minority carriers at equilibrium in 300 K. Is this silicon sample n-type or p-type? B. Later an impurity is added so that the sample has n=5×10
16
cm
3
. Find the Fermi level position with respect to E. What kind of impurity (donor or acceptor) is added? Find the added impurity concentration. C. Plot energy band diagrams for (a) and (b), in two separate graphs.
Answers
As an AI tutor, I'll do my best to help you with your question about carrier concentrations, Fermi level, impurities, and energy band diagrams in a silicon sample. Let's break down the question into different parts.
Part A: Equilibrium Carrier Concentrations and Type of Silicon Sample
In an intrinsic semiconductor, the number of majority carriers (electrons or holes) is equal to the number of minority carriers. At equilibrium, the Fermi level (E_f) lies at the middle of the bandgap, which is 0.4 eV below the intrinsic level (E_1).
To find the carrier concentrations, we need to consider the temperature of 300 K. At this temperature, we can use the following formula:
n = Ni * exp((E_f - E_i) / (k*T))
where:
- n is the carrier concentration
- Ni is the intrinsic carrier concentration (depends on temperature)
- E_i is the intrinsic level energy
- k is Boltzmann's constant (8.617333262145 x 10^-5 eV/K)
- T is the temperature in Kelvin
Since the Fermi level is 0.4 eV below E_1, we can calculate the carrier concentrations for both majority and minority carriers.
1. Majority Carrier Concentration (n):
For an n-type semiconductor, the majority carriers are electrons. To find the electron concentration, we use the above formula with E_f = E_1 - 0.4 eV.
2. Minority Carrier Concentration (p):
For an n-type semiconductor, the minority carriers are holes. To find the hole concentration, we use the above formula with E_f = E_1 - 0.4 eV.
Part B: Fermi Level Position, Impurity Type, and Concentration
In this part, an impurity is added to the silicon sample, making it an n-type semiconductor with a concentration of 5×10^16 cm^3.
1. Fermi Level Position:
The Fermi level position with respect to E_1 depends on the doping concentration. For an n-type semiconductor, the Fermi level moves closer to the conduction band (E_c) due to the excess of electrons. We can calculate the new Fermi level position using the following formula:
E_f = E_c - (k * T * ln(N_d / n_i))
where:
- E_c is the conduction band energy
- N_d is the donor concentration (5×10^16 cm^3)
- n_i is the intrinsic carrier concentration
2. Impurity Type and Concentration:
The impurity added to the silicon sample makes it an n-type semiconductor. In an n-type semiconductor, the impurity is a donor, as it provides extra electrons. The concentration of the added impurity is given as 5×10^16 cm^3.
Part C: Energy Band Diagrams
To plot the energy band diagrams for the two scenarios (a) and (b), we need to illustrate the energy levels of the valence band (E_v), the conduction band (E_c), and the Fermi level (E_f) in two separate graphs. The positions of these energy levels will vary based on the doping concentration and type of impurity.
I hope this explanation helps you understand the different parts of the question. If you have any further questions, feel free to ask.
To know more about tutor visit:
https://brainly.com/question/28275286
#SPJ11
What is the acceleration acquired by an object that has a mass of 50kg and is pushed with a force of 20N.
Answers
If a 20 N force is applied to a 50 kg mass, then the acceleration acquired by the body is 0.4 m/s².
¿How to calculate the acceleration of a body?It is possible to know the acceleration of a body from Newton's second law, which states that the acceleration is defined as:
a = F/mWhere:
A = accelerationF = forceM = massTroubleshooting:We proceed to find the acceleration of the body, such that:
a = F/ma = 20N / 50kga = 0.4m/s²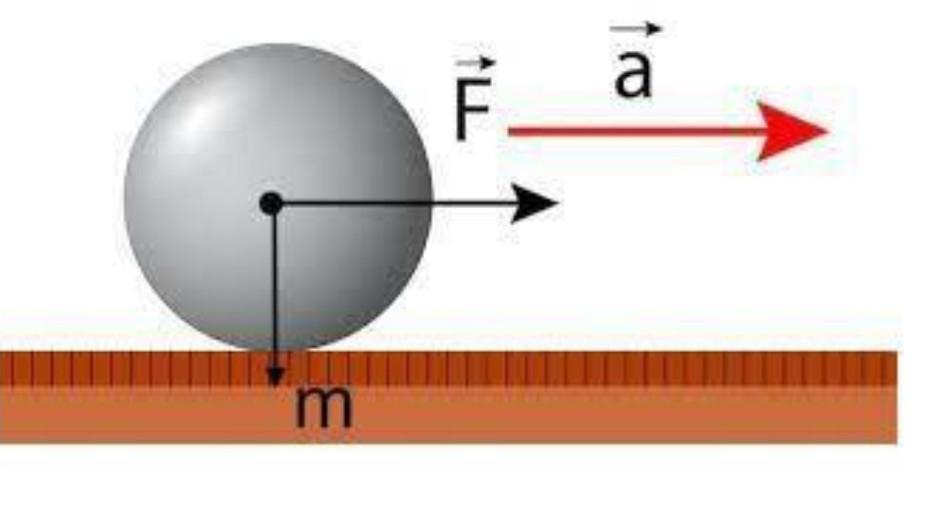
What causes the process of transmutation?
O A. A gamma ray is emitted from an atom's nucleus.
B. Mass is lost due to the formation of a nucleus.
C. Quarks in the nucleus shift between particles.
D. The number of protons in the nucleus changes.
Answers
Answer: D. The number of protons in the nucleus changes.
Explanation:
Transmutation occurs due to a change in the number of protons in the nucleus as this will cause the element to either become another isotope of the same element or an entirely new element altogether.
There are two types of transmutation; Natural and Artificial. Natural transmutation happens as unstable elements such as Uranium-238 try to find stability. They decay until they become a stable element. Artificial transmutation happens when atoms are collided with particles that cause them to change.