A particular saturated solution of Ca3(PO4)2 has [Ca2+]=[PO3?4]=2.9×10?7M. A) What is the value of Ksp for Ca3(PO4)2?
Answers
The Ksp value of \(Ca_3(PO_4)_2\) is \(2.9 * 10^{-14 }M^2.\)
Ksp stands for "equilibrium constant for a speciation reaction." It is a measure of the stability of a solution with respect to the formation of a particular ionic species. Specifically, it is the ratio of the concentrations of the ions that are consumed (or "used up") in forming the ionic species to the concentration of the ionic species itself.
Ksp values are used to predict the relative stability of different ionic species in a solution, and they can be used to predict the concentrations of the ions in a solution as a function of time, given certain conditions. Ksp values can also be used to predict the conditions under which a particular ionic species will be formed or consumed in a reaction
The Ksp value of \(Ca_3(PO_4)_2\) can be calculated using the following equation:
\([Ca_2+][PO_3^4] = Ksp\)
From the given information, we have\([Ca_2+] = 2.9 * 10^{-7 }M, [PO_3^4] = 2.9 * 10^{-7 }M.\)
Therefore, we can substitute these values into the equation and solve for Ksp:
\([Ca__2+][PO3^4] = {(Ksp)} \\\\\\2.9 * 10^{-7} M[Ca_2+] * 2.9 * 10^{-7} M[PO_3^4] = Ksp\\\)
Ksp = \(2.9 * 10^{-14 }M^2.\)
Therefore, the Ksp value of \(Ca_3(PO_4)_2\) is \(2.9 * 10^{-14 }M^2.\)
Learn more about Ksp value visit: brainly.com/question/29610286
#SPJ4
Related Questions
Select all the correct answers.
Which statements are true about all waves?
It’s caused by a disturbance.
It requires a medium to travel.
It carries energy.
It carries matter.
Answers
Answer:
It’s caused by a disturbance.
It requires a medium to travel.
Explanation:
A wave is an unsettling influence that sends energy starting with one point then onto the next. It does no essentially convey the particles or matter of the medium.
Waves typically conveys energy. It is this energy they use in making aggravations.
Not all waves conveys matter; a genuine model is electromagnetic waves. Additionally, electromagnetic waves don't need a mode for proliferation.
Answer: The only answer that is NOT true is that waves carry matter.
Explanation:
Waves are caused by a disturbance, require a medium to travel (by definition) and carry energy.
If you're talking about electromagnetic waves tho, those do not require a medium. Waves in general DO require a medium
100 mL of an NaOH solution is neutralized by 50 mL of a 0.5M HCl solution. What is the morality of the NaOH solution?
Answers
Answer:
0.25M
Explanation:
acording to M1V1/M2V2=n1/n2
what 3 classes of compounds are elecrolytes
Answers
Answer:
i believe it is strong acids, strong bases, and salts.
Explanation:
Hope this helps : )
What volume of 0.250 M sulfuric acid (in milliliters) is needed to react completely with 2.17 g of potassium bicarbonate
Answers
The total volume of 0.250 M sulfuric acid (in milliliters) is needed to react completely with 2.17 g of potassium bicarbonate is 43.4 milliliters approximately.
To determine the volume of 0.250 M sulfuric acid needed to react completely with 2.17 g of potassium bicarbonate, we first need to calculate the moles of potassium bicarbonate. Then, we can use stoichiometry to determine the volume of sulfuric acid.
The molar mass of potassium bicarbonate (KHCO3) is 100.12 g/mol. Therefore, 2.17 g of potassium bicarbonate is equal to 2.17 g / 100.12 g/mol = 0.0217 mol.
The balanced chemical equation for the reaction between sulfuric acid (H2SO4) and potassium bicarbonate is:
H2SO4 + 2KHCO3 -> K2SO4 + 2H2O + 2CO2
From the balanced equation, we can see that one mole of sulfuric acid reacts with two moles of potassium bicarbonate.
Therefore, the number of moles of sulfuric acid required is 0.0217 mol × (1 mol H2SO4 / 2 mol KHCO3) = 0.01085 mol.
Now, we can calculate the volume of 0.250 M sulfuric acid using the equation:
Molarity = moles / volume
0.250 M = 0.01085 mol / (volume in liters)
Solving for the volume in liters:
volume in liters = 0.01085 mol / 0.250 M = 0.0434 L
Finally, we convert the volume from liters to milliliters:
volume in milliliters = 0.0434 L × 1000 mL/L = 43.4 mL
Therefore, approximately 43.4 milliliters of 0.250 M sulfuric acid is needed to react completely with 2.17 g of potassium bicarbonate.
To learn more about, Molarity , click here, https://brainly.com/question/13386686
#SPJ11
On a distance-time graph, at 2 hours the graph is at a height of 20 meters, and at 4 hours it is at a height of 80 meters. The average speed for the interval is 30 meters/hour.
true
false
Answers
Answer:
true
Explanation:
In 2 hours, it went 60 metres. 60 / 2 = 30 so true
convert 8.7x10^24 atoms of ammonium chloride to grams of ammonium cloride
Answers
The mass of 8.7x10^24 atoms of ammonium chloride would be 772.93 grams.
Mass of atoms of substancesAccording to standards, 6.022 x \(10^{23\) atoms of any substance is equivalent to 1 mole of the substance.
6.022 x \(10^{23\) atoms = 1 mole
8.7 x \(10^{24\) atoms of ammonium chloride = 8.7 x \(10^{24\) /6.022 x \(10^{23\)
= 14.45 mol of ammonium chloride.
The molar mass of ammonium chloride is 53.49 g/mol.
The mass of 14.45 mol ammonium chloride would be:
14.45 x 53.49 = 772.93 grams
In other words, 8.7 x \(10^{24\) atoms of ammonium chloride is equivalent to 772.93 grams of ammonium chloride.
More on moles and atoms of substances can be found here: https://brainly.com/question/28864489
#SPJ1
please help me with my homework
5. Identify each of the following as element, compound, homogeneous
mixture, or heterogeneous mixture. Explain your reasoning for each.
a. milk
b. iron nail
c. glass
d. sugar
e. bottled spring water
f. distilled water
g. air
h. alloy bicycle frame
i. propane
i.
baking soda
Answers
Milk : Homogenous
glass : Homogenous
sugar : compounds
bottled spring water : Homogenous
distilled water : compounds
air : Homogenous
alloy bicycle frame : Homogenous
propane : compounds
baking soda : Homogenous
When two liquids totally mix, they produce a homogenous combination.
Complete dissolution of a solid in a liquid
When there is no phase difference in a combination, it is said to be homogenous. The composition of a homogeneous mixture is constant and consistent across the whole system.
A mixture of water and ethanol, for instance, is an example of a homogeneous mixture since the two substances are miscible in all amounts.
To know more about homogenous refer to https://brainly.com/question/16938448
#SPJ9
1000 silver atom weight 1.79×10^-19 g. calculate atomic mass of silver
Answers
Answer:
One million of silver atoms weigh 1.79 xx 10^(-16)g. Calculate the atomic mass of silver. Atomic mass of silver =107.8g=107.8u
electron affinity and ionization energy do not rigorously adhere effective nuclear charge arguments because:
Answers
The capacity of an atom to absorb electrons can be indicated by its electron affinity. An atom gains electrons more readily the lower its first electron affinity. The ability of an atom to gain electrons is weaker the higher the electron affinity. The ionization energy demonstrates an atom's capacity to lose electrons.
What is effective nuclear charge ?
The attractive positive charge of nuclear protons acting on valence electrons is known as effective nuclear charge. Due to the shielding effect, the effective nuclear charge is always smaller than the total amount of protons in a nucleus.
To know about effective nuclear charge from the link
https://brainly.com/question/13647434
#SPJ4
(1) what is the element with an electron configuration of 1s22s22p63s23p64s23d1?
Answers
We can read an electron configuration as if we are reading the periodic table like a book, from left to right, line after line.
There are multiple blocks on the periodic table, including s, p, d and f.
Rows can be indicated by energy levels, which can be different for each block.
Finally, superscripts define which element on that energy level in a block we are referring to. For instance, a superscript of 2 means the second element in that row.
See the image below for a more visual understanding. (Taken from Study.com).
Given this information and the electron configuration \(1s^22s^22p^63s^23p^64s^23d^1\), we can read the periodic table like a book to determine that the element is scandium.

Why do rocks on the ocean floor form a pattern of magnetized stripes? hurry hurry pls pls pls HURRY
Answers
Answer:
BEST ANSWER :The rocks contain iron that points in the direction the rocks move away from the ridge.
Does the sound wave above experience a change in pitch or loudness? Why? (PLS HELP 60 ILL PNTS AND ILL GIVE BRAINIEST!!
Answers
Answer:
Explanation:
Amplitude, or the size of the wave height, affects the loudness. Larger the amplitude, the louder the volume. Period, or the time it takes for the wave to complete one round, determines the pitch. Shorter period (or higher frequency) means higher pitch.
Which solution has the higher boiling point?
(a) 38.0 g of C₃H₈O₃ in 250. g of ethanol or 38.0 g of C₂H₆O₂ in 250. g of ethanol
Answers
The higher boiling point is 38.0 g of C₃H₈O₃ in 250. g of ethanol.
what is ethanol?The chemical compound ethanol, sometimes referred to as ethyl alcohol, grain alcohol, or alcohol, belongs to the family of compounds known as alcohols and has the molecular formula C2H5OH. A crucial industrial chemical known as ethanol is utilised as a gasoline additive, a solvent, and a raw material for the synthesis of other organic molecules (forming a mixture known as a gasohol). Many alcoholic drinks, such as beer, wine, and distilled spirits, contain the intoxicating substance ethanol.
The process used to make alcoholic drinks, carbohydrate fermentation, and ethylene hydration are the two fundamental methods for creating ethanol.
learn more about ethanol refer:
https://brainly.com/question/5750283
#SPJ4
how do you find the LD50 and how do you calculate the amount of substance that would harm a person of a certain weight?
Answers
The LD50 (Lethal Dose 50) is a measure used in toxicology to determine the lethal dose of a substance that would cause death in 50% of the test population.
However, it is important to note that conducting experiments to determine the LD50 of a substance on humans is unethical and illegal. The LD50 values are typically determined through animal testing, usually on rodents such as rats or mice.To calculate the amount of a substance that would harm a person of a certain weight, various factors need to be considered, including the toxicity of the substance and the individual's weight. In toxicology, a commonly used measure is the oral median lethal dose (LD50) expressed as milligrams per kilogram of body weight (mg/kg).To estimate the harmful dose for an individual of a certain weight, you would need to know the LD50 value of the substance and apply it to the weight of the person. The calculation involves multiplying the LD50 value by the person's weight in kilograms. However, it is crucial to emphasize that estimating harmful doses for humans based on animal LD50 values alone can be inaccurate and potentially dangerous.
It is essential to consult professionals in toxicology or poison control centers to obtain accurate information regarding the toxicity of a substance and its potential effects on human health.
for such more questions on substance
https://brainly.com/question/29108029
#SPJ8
How do the ramp heights of the different objects compare? How does the ramp height relate to the strength of the frictional force between the book and the object?
Answers
The height of a ramp does not directly determine the strength of the frictional force between a book and an object.
How do they compare?The strength of the frictional force between a book and an object is not directly influenced by the height of a ramp. The nature of the surfaces in contact, the force forcing the surfaces together (normal force), and the coefficient of friction are some of the variables that affect the frictional force between two surfaces.
The coefficient of friction between the book and the object plays a major role in determining the strength of the frictional force.
Learn more about frictional force:https://brainly.com/question/30280206
#SPJ1
Table sugar has the chemical formula C 12 H 22O 11 what is the ratio of carbon atoms to oxygen atoms in this compound
Answers
In this compound, the proportion of carbon atoms to oxygen atoms is 12: 11.
What distinguishes an atom of carbon as such?
The nucleus of a carbon atom is made up of six protons and six neutrons, and it is encased in six electrons. According to quantum physics, the first two electrons must occupy the innermost atomic orbital, while the wavefunctions of the following four electrons only partially fill the second standard and three second principal orbitals.
How are atoms located in a compound?
Calculate the element's molar mass using the periodic table. To determine the atoms, the number of moles, divide the given mass in grams by the molar mass. To get the number of atoms, multiply the number of moles by Avogadro's number.
To learn more about oxygen atoms click on the link below:
https://brainly.com/question/28108680
#SPJ1
Copper is a transition metal that can have more than one charge. Write the equation of Cu+ reacting with hydrochloric acid. Then write the reaction of Cu+2 reacting with hydrochloric acid. How does the amount of hydrogen gas evolved change with each?
Answers
The amount of hydrogen gas that evolved in the reaction of Cu⁺ with HCl is less than the amount of hydrogen gas that evolved in the reaction of Cu₂⁺ with HCl.
When the Copper (I) ion (Cu⁺) reacts with hydrochloric acid (HCl), it undergoes a single replacement reaction, as follows:
Cu⁺ (aq) + HCl (aq) → CuCl (aq) + H⁺ (aq)
In this reaction, copper (I) ion is oxidized to copper (II) ion (Cu₂⁺) while hydrogen ion (H⁺) is reduced to hydrogen gas (H₂).
The reaction of Copper (II) ion (Cu₂⁺) with hydrochloric acid (HCl) also undergoes a single replacement reaction, as follows:
Cu₂⁺ (aq) + 2HCl (aq) → CuCl₂ (aq) + 2H⁺ (aq)
In this reaction, copper (II) ion is reduced to copper (I) ion (Cu⁺) while hydrogen ion (H⁺) is again reduced to hydrogen gas (H₂).
The second reaction produces twice the amount of hydrogen gas compared to the first reaction.
To learn more about hydrogen follow the link:
https://brainly.com/question/31018544
#SPJ1
which of the following describes the reaction of molecules as snow melts
a) The ice absorbs heat energy and the molecules move further away
b) The ice releases heat energy and the molecules move further away
c) The ice absorbs heat energy and the molecules move closer together
d) The ice releases heat energy and the molecules move closer together
Answers
During the melting of ice, the ice absorbs heat energy and the molecules move further away; option A.
What is melting?Melting refers to the process by which a solid substance changes to liquid when heat is added to it.
The melting of pure substances occur at a definite temperature called the melting point of that substance.
The molecules of a substance move further apart when they melt as the attractive forces between them are weakened.
The melting of ice is an example of the process of melting.
During the melting of ice, the ice absorbs heat energy and the molecules move further away.
In conclusion, melting of solids occur when heat is added to the solid.
Learn more about melting at: https://brainly.com/question/40140
#SPJ1
A mixture is made by mixing alcohol, water and salt then how will you seperate the componen of the mixture?
Answers
Answer: Fractional Distallation
Explanation: this technique relies on the compounds of the mixture. This is able to happen when there is diffrent boilling points.
Which of these is an example of an industry use for radiation?
Answers
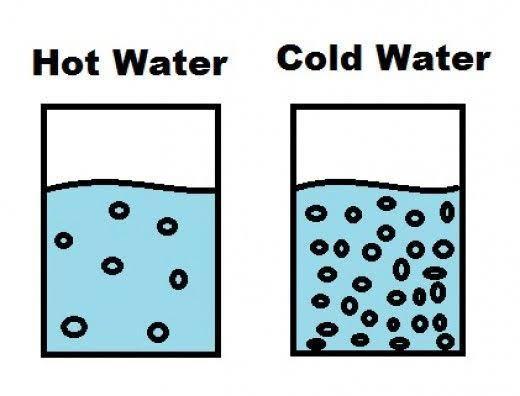
3- You saw a jar of hot water placed upside down over a jar of cold water. The hot
water stayed on top of the cold water without mixing. Why did the hot water stay on
top of the cold water?
Answers
Hot water stays on the top of cold water and doesn't mix. This is because of difference in DENSITY of water at different temperatures.
When we heat water, then the atoms gain energy from the heat and start moving away from each other. Now, when the atoms move away from each other than the (mass per unit volume) i.e. the density decreases. This makes water lighter.
When the temperature is less i.e. cold water then the atoms are more compactly packed and therefore the density of water is more at lower temperatures.
Since, hot water is lighter than cold water that is why it stays on top of the cold water.
Can anybody help me asap pls

Answers
Answer:
Here is ur answer...
Explanation:
2) Half-filled d orbital increases the stability of the element.
Mercury’s atomic emission spectrum is shown below. Estimate the wavelength of the orange line. What is its frequency? What is the energy of a photon corresponding to the orange line emitted by the mercury atom
Answers
The wavelength of the orange line is 610 nm, the frequency of this emission is 4.92 x 10¹⁴ Hz and the energy of the emitted photon corresponding to this orange line is 3.26 x 10⁻¹⁹ J.
"Your question is not complete, it seems to be missing the diagram of the emission spectrum"
the diagram of the emission spectrum has been added.
From the given chart;
The wavelength of the atomic emission corresponding to the orange line is 610 nm = 610 x 10⁻⁹ m
The frequency of this emission is calculated as follows;
c = fλ
where;
c is the speed of light = 3 x 10⁸ m/sf is the frequency of the waveλ is the wavelength\(f = \frac{c}{\lambda } \\\\f = \frac{3\times 10^8}{610 \times 10^{-9}} \\\\f = 4.92 \times 10^{14} \ Hz\)
The energy of the emitted photon corresponding to the orange line is calculated as follows;
E = hf
where;
h is Planck's constant = 6.626 x 10⁻³⁴ JsE = (6.626 x 10⁻³⁴) x (4.92 x 10¹⁴)
E = 3.26 x 10⁻¹⁹ J.
Thus, the wavelength of the orange line is 610 nm, the frequency of this emission is 4.92 x 10¹⁴ Hz and the energy of the emitted photon corresponding to this orange line is 3.26 x 10⁻¹⁹ J.
Learn more here:https://brainly.com/question/15962928

at sea level, what percentage of the air we breathe is oxygen?
Answers
At sea level, the air we breathe is 21% oxygen. This means that for every 100 molecules of air we inhale, 21 of them are oxygen molecules.
The remaining 79% is mostly nitrogen, with small amounts of other gases such as argon and carbon dioxide. The percentage of oxygen in the air can vary slightly depending on factors such as altitude and location.
For example, at higher altitudes, the percentage of oxygen decreases because there is less atmospheric pressure.
Additionally, air pollution can also impact the percentage of oxygen in the air. It is important for our bodies to receive this essential gas to function properly, as oxygen is necessary for cellular respiration and energy production.
At sea level, the percentage of oxygen in the air we breathe is approximately 21%. The air is primarily composed of nitrogen (around 78%), oxygen, and trace amounts of other gases such as argon, carbon dioxide, and water vapor.
The composition remains relatively consistent throughout the lower atmosphere, ensuring that we have a sufficient supply of oxygen to sustain life at varying altitudes.
However, air pressure does decrease with elevation, which can affect the availability of oxygen in higher altitudes.
To know more about oxygen. please visit.....
brainly.com/question/30545420
#SPJ11
what atom has a greater number of neutrons
Answers
Answer:
livermorium
Explanation:
The atom with the largest number of neutrons is a tie between livermorium and tennessine. Each of these atoms contain 177 neutrons.
This model uses a red pin to show one focus of the ellipse. In the real-world, what solar system object would take the place of the pin?

Answers
The Sun would take the place of the pin in the real-world, as it is the focus of the elliptical orbits of the planets in our Solar System.
What is Solar System?Solar System is a collection of celestial objects that orbit around the sun such as planets, moons, asteroids, comets, and meteoroids. It consists of the sun and everything that orbits it, including all of the planets, moons, asteroids, comets, and meteoroids. The planets and other objects in the Solar System are held in their orbits by the gravity of the sun. The sun is the most important source of energy for the Solar System, providing light and heat for the planets. The planets and other objects in the Solar System are arranged in a specific order, with the sun at the center. The four inner planets are known as the rocky planets, consisting of Mercury, Venus, Earth, and Mars. The four outer planets are known as the gas giants, consisting of Jupiter, Saturn, Uranus, and Neptune. The asteroid belt lies between Mars and Jupiter, while the Kuiper belt lies beyond Neptune.
To learn more about solar system
https://brainly.com/question/28599133
#SPJ1
Devise a 6-step synthesis of a carboxylic acid from ethyne using the reagents provided.
Answers
A synthesis reaction is one in which a product is made from two or mote reactants.
What is reaction mechanism?The term reaction mechanism refers to the sequence of steps that has to be involved as a reactant is converted to products.
The process of converting ethyne to carboxyllic acid passes through a six step process which includes the oxidation of the substrate using permanganate in basic solution.
The detailed mechanism for the synthesis of a carboxylic acid from ethyne is shown in the image attached to this answer.
Learn more about synthesis reaction: https://brainly.com/question/24936069

Determine whether each property is a physical property or a chemical property.
Solubility
Choose...Physical propertyChemical property
Flammability
Choose...Physical propertyChemical property
Reactivity
Choose...Physical propertyChemical property
Color
Choose...Physical propertyChemical property
Density
Choose...Physical propertyChemical property
Melting point
Choose...Physical propertyChemical property
Answers
Here are the given properties. The task is to decide whether each property is a physical property or a chemical property:
1. Solubility: Physical property
2. Flammability: Chemical property
3. Reactivity: Chemical property
4. Color: Physical property
5. Density: Physical property
6. Melting point: Physical property
There are two types of properties: Physical property and Chemical property. In this task, we have to identify which property among Solubility, Flammability, Reactivity, Color, Density, Melting point is Physical property or Chemical property.
A Physical property is a characteristic that defines the behavior or appearance of a substance without any changes in its composition. Physical properties can be identified by measuring, observing, or using physical methods.
A Chemical property is a characteristic that defines the behavior or the ability of a substance to undergo a chemical change or to transform into a new substance. The correct answer is given above.
To learn more about "physical property", visit: https://brainly.com/question/12330204
#SPJ11
an unknown compound contains only c , h , and o . combustion of 3.70 g of this compound produced 8.71 g co2 and 2.38 g h2o . what is the empirical formula of the unknown compound? insert subscripts as needed.
Answers
The empirical formula of the unknown compound is CH.
The empirical formula of a compound represents the simplest whole number ratio of atoms in that compound. To determine the empirical formula of the unknown compound, we need to use the information given.
First, let's calculate the moles of CO2 produced. The molar mass of CO2 is 44.01 g/mol, so:
moles of CO2 = mass of CO2 / molar mass of CO2
moles of CO2 = 8.71 g / 44.01 g/mol
moles of CO2 = 0.1979 mol
Next, let's calculate the moles of H2O produced. The molar mass of H2O is 18.02 g/mol, so:
moles of H2O = mass of H2O / molar mass of H2O
moles of H2O = 2.38 g / 18.02 g/mol
moles of H2O = 0.132 mol
Now, let's calculate the moles of carbon and hydrogen in the unknown compound. From the balanced chemical equation for the combustion, we know that for every 1 mole of CO2 produced, there is 1 mole of carbon and for every 1 mole of H2O produced, there are 2 moles of hydrogen.
moles of C = moles of CO2
moles of C = 0.1979 mol
moles of H = 2 * moles of H2O
moles of H = 2 * 0.132 mol
moles of H = 0.264 mol
Now, let's calculate the moles of oxygen in the unknown compound. Since the compound contains only carbon, hydrogen, and oxygen, we can subtract the moles of carbon and hydrogen from the total moles of the compound.
moles of O = total moles - moles of C - moles of H
moles of O = 0.1979 mol + 0.264 mol - total moles
moles of O = 0.4629 mol - total moles
Given that the mass of the unknown compound is 3.70 g, we can calculate the moles of the compound using its molar mass.
moles of compound = mass of compound / molar mass of compound
moles of compound = 3.70 g / molar mass of compound
Since we are looking for the empirical formula, we can assume a convenient mass for the unknown compound, such as 100 g. This will make it easier to find the empirical formula.
moles of compound = 3.70 g / molar mass of compound
moles of compound = 100 g / molar mass of compound
Now, we can set up an equation to find the molar ratio of carbon, hydrogen, and oxygen in the compound:
0.1979 mol / moles of compound = moles of C / moles of compound
0.264 mol / moles of compound = moles of H / moles of compound
0.4629 mol - moles of compound / moles of compound = moles of O / moles of compound
Simplifying these equations, we get:
0.1979 = moles of C
0.264 = moles of H
0.4629 - moles of compound = moles of O
Since we assumed a mass of 100 g for the compound, we can convert the moles to grams:
0.1979 mol = 0.1979 * molar mass of C
0.264 mol = 0.264 * molar mass of H
0.4629 - moles of compound = 0.4629 * molar mass of O
Now, we can find the molar masses of carbon, hydrogen, and oxygen. The molar mass of carbon is 12.01 g/mol, the molar mass of hydrogen is 1.008 g/mol, and the molar mass of oxygen is 16.00 g/mol.
0.1979 * molar mass of C = 0.1979 * 12.01 g/mol
0.264 * molar mass of H = 0.264 * 1.008 g/mol
0.4629 - moles of compound * molar mass of O = 0.4629 * 16.00 g/mol - moles of compound
Now, let's simplify and solve for the molar masses:
2.373979 g = 2.521712 g - moles of compound
moles of compound = 0.147733 g
Now, let's substitute the moles of carbon, hydrogen, and oxygen into the equation:
0.1979 = 0.1979 / 0.147733
0.264 = 0.264 / 0.147733
0.4629 - moles of compound = 0.4629 - 0.147733
Simplifying these equations, we get:
1 = 1
1 = 1
0 = 0
This means that the ratio of carbon, hydrogen, and oxygen in the unknown compound is 1:1:0. Therefore, the empirical formula of the unknown compound is CH.
Learn more about empirical formula here:-
https://brainly.com/question/32125056
#SPJ11
4
The thermometer used by Student A was indicating temperatures that were 5 °C higher
than the thermometer used by Student B when measuring the same solutions. Would
this affect the E, values obtained by the two students? Explain.
Answers
Answer:
Yes.
Explanation:
Yes, this difference of readings will definitely affect the results of the experiment as well as the E values because the readings taken by both students are different from one another. There is a fault in one of the thermometer because both shows different readings of temperature of the same solution. This will affect the overall experiment and due to this error, we are unable to tell that which one reading is correct so the answer is uncertain or unsure.