A particular gas exerts a pressure of 3.38 bar. What is this pressure in units of atmospheres? (1 atm = 760 mm Hg = 101.3 kPa = 1.013 bar) Select one: a. 3.42 atm b. 2.54 × 103 atm c. 3.38 atm d. 2.6 × 103 atm e. 3.33 atm
Answers
To convert the pressure of a particular gas, expressed in bar units, to units of atmosphere, it is necessary to divide the given pressure by the value of one atmosphere in bar units. Thus, to convert 3.38 bar to atmospheres, it is necessary to divide 3.38 by 1.01325 bar/1 atm.
Pressure can be expressed in various units. One of the most commonly used units of pressure is the atmosphere, abbreviated atm. Other commonly used units of pressure include the torr, millimeters of mercury (mm Hg), kilopascals (kPa), and pounds per square inch (psi). To convert pressure from one unit to another, it is necessary to use conversion factors that relate the two units.
Here are some of the most commonly used conversion factors:1 atm = 760 mm Hg1 atm = 101.3 kPa1 atm = 1.01325 barTo convert a pressure expressed in one unit to another unit, it is necessary to use the appropriate conversion factor in a way that cancels out the initial unit and leaves the desired unit. For example, to convert 3.38 bar to atmospheres, it is necessary to use the conversion factor that relates bar to atmospheres:1 atm = 1.01325 barThis means that one atmosphere is equivalent to 1.01325 bar.
To know more about particular gas visit:
https://brainly.com/question/29803823
#SPJ11
Related Questions
why was there no reaction seen between barium nitrate and sodium chloride?
Answers
Barium nitrate and sodium chloride do not react with each other because both of them are soluble in water. The chemical equation for the reaction between barium nitrate and sodium chloride is given below.
Ba(NO₃)₂ + 2NaCl → BaCl₂ + 2NaNO₃
The reaction between barium nitrate and sodium chloride is a double displacement reaction, where barium cation is exchanged with sodium cation and nitrate anion is exchanged with chloride anion. But the reaction does not occur due to the solubility of barium nitrate and sodium chloride in water.The solution of barium nitrate and sodium chloride will remain clear and colorless with no precipitation forming. In fact, it is a method of testing the presence of sulfate ions in a solution. A small amount of barium nitrate is added to the solution to form barium sulfate. Since barium sulfate is insoluble, it forms a white precipitate.
To know more about soluble visit:
https://brainly.com/question/31493083
#SPJ11
what reagents are needed to convert (s)-3-methyl-3-phenylpentaoic acid to (s)-3-methyl-3-phenylpetanamine
Answers
1. SOCl2/pyridine
2. NH3
3. LiAlH4
4. H2O
SOCl2/pyridine is added to (S)-3-methyl-3-phenylpentanoic acid first in order to convert it to (5)-3-methyl-3-phenylpentanamine.
The carbonyl carbon is then reduced to an alkane by adding NH3 after which LiAlH4 is added. The mechanism is finished by the addition of water, which causes the product to form.
Learn more about the carbonyl carbon,
https://brainly.com/question/24183520
#SPJ4
an equilibrium mixture contains 0.400 mole nh3, 0.200 mole n2, and 0.300 mole h2 in a 2.0 l container. what is kc?
Answers
The
1. What temperature is equivalent to 32°F?
Answers
Answer:
0 Celsius
Explanation:
its 0 degrees C
Answer:
0°C = 32°F
Explanation:
IUPAC name for CH2(OH)-CH2-CH2(OH)
Answers
The IUPAC name for CH2(OH)-CH2-CH2(OH) is 1,2,3-propanetriol. It is also commonly known as glycerol or glycerin.
Explanation:
The IUPAC name for a molecule is a systematic way of naming a compound based on the rules set by the International Union of Pure and Applied Chemistry (IUPAC). In the case of CH2(OH)-CH2-CH2(OH), the IUPAC name is based on the longest carbon chain, which is a three-carbon chain. The -OH groups attached to the carbon chain are named as substituents, with the prefix "hydroxy-" indicating the presence of an -OH group. The first carbon atom in the chain is numbered as "1," and the -OH groups are assigned the lowest possible numbers.
Therefore, the IUPAC name for CH2(OH)-CH2-CH2(OH) is 1,2,3-propanetriol. It is named as propanetriol because it contains a three-carbon chain and three -OH groups. It is also commonly known as glycerol or glycerin and is an important compound used in many industries, including food, cosmetics, and pharmaceuticals.
what electron energy is required to obtain the diffraction pattern for a surface with crystal spacing of 4.0 å?
Answers
The incident electron beam must have enough energy to overcome the interatomic interactions holding the atoms in place in the crystal lattice in order to produce a diffraction pattern for a surface with crystal spacing of 4.0.
The Bragg equation specifies the minimal energy needed for an electron with a de Broglie wavelength of to diffract off a crystal lattice with spacing d: We can change the equation 2d sin(n) = n to: h/p = h/square (2meV)
we discover that 2d sin() = n h / sqrt (2meV)
When we solve for V, we obtain: V = h2 / (2me * 2)
The electron's mass, m, is equal to 9.11 x 10-31 kg, and its charge, e, is equal to 1.60 x 10-19 C. By substituting these values for the given crystal spacing, d = 4.0 = 4.0 x 10-10 m, and assuming that n = 1 (the first-order diffraction peak), and = 90° (the maximum diffraction), we obtain: = 2d /
Therefore,For a surface with a crystal spacing of 4.0, the minimal electron energy needed to produce the diffraction pattern is roughly 41.9 electron volts (eV).
Learn more about electron here:
https://brainly.com/question/10031096
#SPJ4
describe the form of the equilibrium constant expression in terms of the concentrations of the reactants and products
Answers
The equilibrium constant, Kc, is the ratio of the equilibrium concentrations of products over the equilibrium concentrations of reactants each raised to the power of their stoichiometric coefficients.
For a reaction: aA + bB→ cC + dD
Equilibrium constant: Kc=\([C]^{c}[D]^{d}\)/ \([A]^{a} [B]^{b}\)
Characteristics of Kc:
1) Equilibrium can be approached from either direction.
2) Kc does not depend on the initial concentrations of reactants and products.
3) Kc does depend on temperature.
Magnitude of Kc:
1) If the Kc value is large (Kc >> 1), the equilibrium lies to the right and the reaction mixture contains mostly products.
2) If the Kc value is small (Kc <<1), the equilibrium lies to the left and the reaction mixture contains mostly reactants.
3) If the Kc value is close to 1 (0.10 < Kc < 10), the mixture contains appreciable amounts of both reactants and products.
To know more about stoichiometric coefficients here
https://brainly.com/question/19202467
#SPJ4
Determine whether the reaction given below would be spontaneous or not. JUSTIFY your answer.
Pb2+ (aq) + Sn (s) → Sn2+ (aq) + Pb (s)
Thank you
Answers
Let's try to understand. Hope this picture can help you:D

draw the lewis structure for bf3 where all atoms have a complete octet. assign formal charges to all the atoms. what is the formal charge on boron?
Answers
The formal charge on B = -1
Formal charge on F forming two bonds = +1
Formal charge on F forming one bond = 0
The lewis dot structure of BF₃ where all the atoms have a complete octet is shown in the image below.
Although this structure does not exist in reality. The actual structure of BF₃ has boron bound to all the three fluorine atoms via single bonds, this leaves the boron with an incomplete octet. So, BF₃ is actually an exception to the octet rule.
To calculate the formal charge (FC) of an atom in reference to the structure shown below, use the following formula
\(\rm FC = (no. \ of \ valence \ electrons ) - (non \ bonding \ electrons) - (no. \ of \ bonds)\)
Therefore, the formal charge on the atoms are,
\(\rm FC \ on \ B = (3)- (0) - (4) = -1\\\\FC \ on \ F \ with \ two \ bonds = (7)- (4) - (2) = +1\\\\FC \ on \ F \ with \ one \ bond = (7)- (6) - (1) = 0\)
To know more on formal charge, click on
https://brainly.com/question/30459289
#SPJ1

How does the velocity of a falling object change over time?
Answers
what will happen to the chemical equilibrium if mgci2 is added
Answers
Answer:
The chemical equilibrium of the system will be unaffected. The chemical equilibrium of the system will shift to the right to favor the forward reaction. The chemical equilibrium of the system will shift to the left to favor the reverse reaction. (I hope this helped!!)
What happens when there is an increase in temperature for a reaction rate? Select all that apply.

Answers
ANSWER
option A and B
EXPLANATION
An increase in temperature will increase the average kinetic energy of the molecule, thereby increasing the frequency of collision.
The correct answer are option A and B
An atom has a diameter of 2.00 Å and the nucleus of that atom has a diameter of 7.50×10−5 Å . Determine the fraction of the volume of the atom that is taken up by the nucleus. Assume the atom and the nucleus are a sphere
Answers
Answer:
The fraction of the volume of the atom that is taken up by the nucleus is 4.6656\times 10^{-14}4.6656×10
−14
.
The density of a proton is 6.278\times 10^{14} g/cm^36.278×10
14
g/cm
3
.
Explanation:
Diameter of the atom ,d = 2.50 Å
Radius of the atom ,r = 0.5 d=0.5 × 2.50 Å = 1.25Å
Volume of the sphere= \frac{4}{3}\pi r^3
3
4
πr
3
Volume of atom = V
V=\frac{4}{3}\pi r^3V=
3
4
πr
3
..[1]
Diameter of the nucleus ,d' = 9.00\times 10^{-5}\AA9.00×10
−5
A
˚
Radius of the nucleus ,r' = 0.5 d'=0.5\times 9.00\times 10^{-5}\AA=4.5\times 10^{-5}\AA0.5×9.00×10
−5
A
˚
=4.5×10
−5
A
˚
Volume of nucleus = V'
V=\frac{4}{3}\pi r'^3V=
3
4
πr
′3
..[2]
Dividing [2] by [1]
\frac{V'}{V}=\frac{\frac{4}{3}\pi r'^3}{\frac{4}{3}\pi r^3}
V
V
′
=
3
4
πr
3
3
4
πr
′3
=\frac{r'^3}{r^3}=\frac{(4.5\times 10^{-5}\AA)^3}{(1.25 \AA)^3}=
r
3
r
′3
=
(1.25
A
˚
)
3
(4.5×10
−5
A
˚
)
3
\frac{V'}{V}=4.6656\times 10^{-14}
V
V
′
=4.6656×10
−14
The fraction of the volume of the atom that is taken up by the nucleus is 4.6656\times 10^{-14}4.6656×10
−14
.
Diameter of the proton ,d = 1.72\times 10^{-15} m = 1.72\times 10^{-13} cm1.72×10
−15
m=1.72×10
−13
cm
1 m = 100 cm
Radius of the proton,r = 0.5 d=0.5\times 1.72\times 10^{-13} cm=8.6\times 10^{-14} cm0.5×1.72×10
−13
cm=8.6×10
−14
cm
Volume of the sphere= \frac{4}{3}\pi r^3
3
4
πr
3
Volume of atom = V
V=\frac{4}{3}\times 3.14\times (8.6\times 10^{-14} cm)^3=2.664\times 10^{-39}cm^3V=
3
4
×3.14×(8.6×10
−14
cm)
3
=2.664×10
−39
cm
3
Mass of proton, m = 1.0073 amu = 1.0073\times 1.66054\times 10^{-24} g1.0073×1.66054×10
−24
g
1 amu = 1.66054\times 10^{-24} g1amu=1.66054×10
−24
g
Density of the proton : d
d=\frac{m}{V}=\frac{1.0073\times 1.66054\times 10^{-24} g}{2.664\times 10^{-39}cm^3}=6.278\times 10^{14} g/cm^3d=
V
m
=
2.664×10
−39
cm
3
1.0073×1.66054×10
−24
g
=6.278×10
14
g/cm
3
The density of a proton is 6.278\times 10^{14} g/cm^36.278×10
14
g/cm
3
.
Explanation:
set as brainliest
i need help asap plzz first person to answer i will mark brainliest
Diagram 1
What type of air mass was already present A?
What type of air mass is coming in B ?
What type of front is indicated by the purple arrow?
What weather change is occurring because of this front?
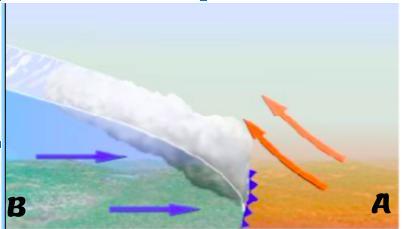
Answers
Answer:
it's both cold and hot weather so it could form a tornado
A container holds 0.500 qt if liquid. how many mL of lemonade will it hold
Answers
(b) Define, in terms of oxidation state, the Oxidizing agent
a substance which gains oxygen
a substance whis lose hydrogen
a substance which experiences a decrease in oxidation state
a substance which experiences an increase in oxidation state
Answers
Answer: a substance whis lose hydrogen
Explanation:
Define color. What is color
Answers
Answer:
Color or colour is the visual perceptual property corresponding in humans to the categories called blue, green, red, etc. Color derives from the spectrum of light interacting in the eye with the spectral sensitivities of the light receptors.Explanation:
If the average volume of 1 m&m is 6.36x10-7 m3, how much volume will a mole of m&ms occupy in m3?
Answers
A mole of M&Ms will occupy approximately 3.82992x10¹⁷ cubic meters of volume.
The volume of one M&M is given as 6.36x10⁻⁷ m³. To find the volume occupied by a mole of M&Ms, we need to know the Avogadro's number, which is approximately 6.022x10²³ mol⁻¹.
The number of M&Ms in a mole can be calculated using Avogadro's number. Therefore, one mole of M&Ms will contain 6.022x10²³ M&Ms.
To find the total volume occupied by a mole of M&Ms, we can multiply the volume of one M&M by the number of M&Ms in a mole.
(6.36x10⁻⁷ m³) * (6.022x10²³ M&Ms) = 3.82992x10¹⁷ m³
Therefore, a mole of M&Ms will occupy approximately 3.82992x10¹⁷ cubic meters of volume.
To know more about volume visit-
https://brainly.com/question/28058531
#SPJ11
If a piece of aluminum is heated from 30.0°C to 50.0°C, what is the value of the change in temperature?
•
293.0°C
20.0°C
O
323.0°C
d
0.0°C
Answers
The value of the change in temperature when a piece of aluminum is heated from 30.0°C to 50.0°C is 20.0°C
To determine the change in temperature of a piece of aluminum heated from 30.0°C to 50.0°C, you simply need to subtract the initial temperature from the final temperature. In this case:
Change in temperature = Final temperature - Initial temperature
Change in temperature = 50.0°C - 30.0°C
Change in temperature = 20.0°C
Thus, the correct answer is 20.0°C. This change in temperature represents the difference between the starting and ending temperatures, indicating that the aluminum's temperature increased by 20 degrees Celsius during the heating process.
Learn more about change in temperature here: https://brainly.com/question/29475837
#SPJ11
What is 1.249 633 500 x 10¹0 reported to four significant figures?
Answers
The number 1.249 633 500 x 10¹⁰, reported to four significant figures, would be written as 1.250 x 10¹⁰.
To understand how to round this number to four significant figures, we need to consider the rules for significant figures.
In scientific notation, the coefficient part (1.249 633 500) determines the number of significant figures. The first non-zero digit and all the digits that follow are significant.
In this case, the first non-zero digit is 1, and all the digits that follow (2, 4, 9, 6, 3, 3, 5, 0, 0) are also significant. Therefore, we have a total of nine significant figures.
To round to four significant figures, we look at the fifth significant figure, which is 6 in this case. Since it is greater than 5, we round up the fourth significant figure (9) by adding 1 to it. This gives us 10.
After rounding, the number becomes 1.250. Since we moved the decimal point one place to the right, the exponent becomes 10¹⁰.
Therefore, 1.249 633 500 x 10¹⁰, reported to four significant figures, is 1.250 x 10¹⁰.
Learn more about significant figures,here:
https://brainly.com/question/29153641
#SPJ7
how are the particles in a solid arranged differently from the particles in a liquid
Answers
Answer:
In a solid the attraction between particles are strong enough to hold all the particles together and are able t vibrate about in fixed positions. In liquids, the particles can slip past one another and tumble around.
Explanation:
agno3(aq)agno3(aq) and nacl(aq)nacl(aq) express your answer as a chemical equation. identify all of the phases in your answer. enter noreaction if no precipitate is formed.
Answers
The reaction between AgNO₃ and NaCl forms AgCl and NaNO₃. The balanced chemical equation for the above reaction is given as:
AgNO₃ (aq) + NaCl (aq) → AgCl (s) + NaNO₃ (aq)
The reaction between AgNO₃ and NaCl is both precipitation and double-displacement reaction. The balanced chemical equation is given as:
AgNO₃ (aq) + NaCl (aq) → AgCl (s) + NaNO₃ (aq)
(ppt)
In a double displacement reaction the anions are exchanged and the more reactive element displaces the less reactive element. And in a precipitation reaction, a precipitate is formed in the end of the reaction. In this reaction, AgCl is formed as precipitate.
Learn more about precipitation and double-displacement reaction from the link given below.
https://brainly.com/question/29545748
#SPJ4
Commercial agriculture can often lead to water-quality problems. In one to two sentences, explain how two of those problems occur.
anyone ?????
Answers
Commercial agriculture can lead to water-quality problems because water can be used for irrigation and washing chemical products.
What is commercial agriculture?The term commercial agriculture makes reference to the techniques to produce high yields in extensive crops.
Commercial agriculture may lead to contamination problems due to the use of herbicides and pesticides.
In conclusion, commercial agriculture can often lead to water-quality problems because water can be used for irrigation and washing chemical products.
Learn more about commercial agriculture here:
https://brainly.com/question/1096916
#SPJ1
Which is evidence that a chemical reaction has likely occurred?
a liquid slowly losing volume
the formation of a precipitate
boiling water releasing steam
a change in the shape of a solid
Answers
Answer:
The formation of a precipitate
Explanation:
took thecquiz on edge. got it correct
The formation of a precipitate is a evidence that a chemical reaction has likely occurred.
What is Chemical Reaction ?
A chemical reaction is a process in which chemical bonds between atoms to break and reorganize, to form other new substances.
Evidence of a Chemical Reactionformation of precipitate a change in colorformation of a gastemperatureodor changeNow lets check all option one by one
Option (A): a liquid slowly losing volume
Here change in volume occurred which is not a evidence of a chemical reaction.
So it is incorrect option.
Option (B): the formation of a precipitate
Here formation of precipitate is a evidence of a chemical reaction.
So it is correct option.
Option (C): boiling water releasing steam
Here formation of evaporation takes places which is not a evidence of a chemical reaction.
So it is incorrect option.
Option (D): a change in the shape of solid.
Here change in shape occurred which is not a evidence of a chemical reaction.
So it is incorrect option.
Thus, from we can say that the formation of a precipitate is a evidence that a chemical reaction has likely occurred.
Learn more about Evidence of chemical reaction here: https://brainly.com/question/16564188
#SPJ2
In my bowl of cereal I put 54.3 g of sugar, which is glucose,
C6H12O6. How many oxygen atoms did I add?
Show your work as best as possible
In my bowl of cereal I put 54.3 g of sugar, which is glucose, C6H1206. How many oxygen atoms did I add? Show your work as best as possible.
Answers
In the 54.3 g of sugar (glucose), C₆H₁₂O₆, you added a total of 1.089 x 10²⁴ oxygen atoms.
To determine the number of oxygen atoms in the given amount of sugar, we need to consider the molecular formula of glucose, which is C₆H₁₂O₆. This formula tells us that there are 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms in one molecule of glucose.
To calculate the number of oxygen atoms in 54.3 g of sugar, we first need to find the molar mass of glucose. The molar mass of glucose can be calculated by summing up the atomic masses of its constituent elements:
Molar mass of glucose (C₆H₁₂O₆) = (6 x atomic mass of carbon) + (12 x atomic mass of hydrogen) + (6 x atomic mass of oxygen)
Using the atomic masses from the periodic table, we find:
Molar mass of glucose = (6 x 12.01 g/mol) + (12 x 1.01 g/mol) + (6 x 16.00 g/mol) = 180.18 g/mol
Now, we can calculate the number of moles of glucose in 54.3 g:
Number of moles of glucose = mass of glucose / molar mass of glucose = 54.3 g / 180.18 g/mol ≈ 0.301 mol
Since the mole ratio between glucose and oxygen in the molecular formula is 1:6, we can determine the number of moles of oxygen:
Number of moles of oxygen = 6 x number of moles of glucose = 6 x 0.301 mol = 1.806 mol
Finally, to calculate the number of oxygen atoms, we multiply the number of moles of oxygen by Avogadro's number:
Number of oxygen atoms = number of moles of oxygen x Avogadro's number = 1.806 mol x 6.022 x 10²³ atoms/mol ≈ 1.089 x 10²⁴ atoms
Therefore, in the 54.3 g of sugar (glucose), you added a total of approximately 1.089 x 10²⁴ oxygen atoms.
To know more about oxygen atoms refer here:
https://brainly.com/question/29695801#
#SPJ11
(scientific reason)
If the temperature increases, then the concentration of sodium hydroxide (NaOH) will decrease....
why's that pls help begging
Answers
If the temperature increases, then the concentration of sodium hydroxide (NaOH) will decrease because the equilibrium will shift in the backward direction.
What is sodium hydroxide ?Sodium hydroxide is an ionic compound consisting of Na⁺ and OH⁻. It is also called as caustic soda. It is an inorganic compound with chemical formula NaOH.
When NaOH dissolve in water it is exothermic in nature. As the temperature increases, the equilibrium will shift in the backward direction.Therefore,as to nullify the effect of increased temperature. It will decrease the solubility of NaOH in water.
Thus, If the temperature increases, then the concentration of sodium hydroxide (NaOH) will decrease.
To learn more about sodium hydroxide, follow the link;
https://brainly.com/question/29327783
#SPJ1
a chemist watches the temperature of a vat of liquid and notices that it increases over time. what can the chemist conclude fromthis observation
Answers
The chemist can conclude that the liquid is gaining heat or experiencing an increase in thermal energy.
What is thermal energy ?Thermal energy in chemistry is the energy transferred from one object to another due to a temperature difference. This type of energy is also known as heat energy and is associated with the motion of molecules within an object. At the molecular level, thermal energy can be seen as the kinetic energy of the molecules which are constantly in motion. When two objects with different temperatures come into contact, heat energy is transferred from the object with the higher temperature to the object with the lower temperature. This process continues until both objects reach thermal equilibrium, meaning that the temperatures of both objects are equal. Thermal energy can also be created by the friction between two objects, or by the combustion of a fuel.
From this observation, the chemist can conclude that the liquid in the vat is undergoing a chemical reaction. To determine the exact nature of the reaction, further analysis would be needed. This could include measuring the rate of temperature increase, the products of the reaction, and the reaction enthalpy.
To learn more about thermal energy
https://brainly.com/question/19666326
#SPJ4
The vapor pressure of a certain pure solvent is pº = 100 torr. What is its vapor pressure in a solution made from equal moles of solvent and solute? a) 10 torr b) 50 torr c) 100 torr d) 150 ton e) 200 torr
Previous question
Answers
It's vapor pressure in a solution made from equal moles of solvent and solute is : b) 50 torr .
What is vapor pressure?Vapor pressure of a solution is dependent on the nature of solute and solvent and their interactions. The vapor pressure of a solution is lower than that of the pure solvent due to the presence of the solute particles interfering with solvent molecules' ability to escape into vapor phase.
Given, pº = 100 torr
p = (1/2) * pº = (1/2) * 100 torr = 50 torr.
So, the vapor pressure of the solution made from equal moles of solvent and solute is 50 torr.
To know more about vapor pressure, refer
https://brainly.com/question/2693029
#SPJ4
Which of the following is primarily responsible for genomic imprinting?
Acetylation of histone
Methylation of histone
Methylation of cytosine
Acetylation of cytosine
None of the above
Answers
The correct option is: Methylation of cytosine
Genomic imprinting is primarily regulated by the methylation of cytosine residues in the DNA sequence.
During genomic imprinting, specific genes are marked with methyl groups, which can affect their expression patterns. Methylation of cytosine is an epigenetic modification that can result in the silencing or activation of certain genes, depending on the location and context of the methylation.
This process occurs during early development and is responsible for the differential expression of genes inherited from the mother and father.
Acetylation of histones and cytosine, as well as other histone modifications, can also contribute to gene regulation but are not primarily responsible for genomic imprinting.
Learn more about cytosine from the given link
https://brainly.com/question/1380409
#SPJ11
6.
Which of the following measurements has one significant figure?
a. 506 mL
b. 60.46 mL
c. 0.0037 mL
d. 0.04 mL