a molecule with the formula has a trigonal planar geometry. many electron groups are on the central atom?
Answers
In a molecule with a trigonal planar geometry, there are three electron groups on the central atom.
If a molecule with the formula has a trigonal planar geometry, it means that the central atom is surrounded by three electron groups, which are arranged in a flat, triangular shape. These electron groups could be lone pairs or bonded atoms, and they are all in the same plane. Therefore, we can conclude that there are three electron groups on the central atom in this molecule.
Learn more about trigonal planar geometry: https://brainly.com/question/29476210
#SPJ11
Related Questions
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
100g zinc telluride reacts with 100g silver chlorate. How many grams of silver telluride will be produced
Answers
The reaction of 100g zinc telluride with 100g silver chlorate will produce 314.04 grams of silver telluride.
The balanced equation for the reaction between zinc telluride (ZnTe) and silver chlorate (AgClO3) is as follows:
ZnTe + 2AgClO3 ⟶ Zn(ClO3)2 + 2AgTe
The balanced equation shows that for every 1 mole of zinc telluride, 2 moles of silver telluride will be produced. Therefore, we need to convert the masses of zinc telluride and silver chlorate to moles and then use stoichiometry to determine the mass of silver telluride produced.
To calculate the moles of zinc telluride and silver chlorate, we divide their respective masses by their molar masses.
For zinc telluride:
Mass of ZnTe = 100g
Molar mass of ZnTe = 208.04g/mol
Number of moles of ZnTe = 100g / 208.04g/mol = 0.48 mol
For silver chlorate:
Mass of AgClO3 = 100g
Molar mass of AgClO3 = 207.81g/mol
Number of moles of AgClO3 = 100g / 207.81g/mol = 0.48 mol
From the balanced equation, we see that the mole ratio of zinc telluride to silver telluride is 1:2. Therefore, the number of moles of silver telluride produced is twice the number of moles of zinc telluride used.
Number of moles of AgTe produced = 2 × 0.48 mol = 0.96 mol
To calculate the mass of silver telluride produced, we multiply the number of moles by its molar mass.
Mass of AgTe produced = Number of moles × Molar mass
= 0.96 mol × 327.23 g/mol = 314.04 g
Therefore, 314.04 grams of silver telluride will be produced in the reaction.
In conclusion, by using the balanced chemical equation, stoichiometry, and mole calculations, we determined that the reaction of 100g zinc telluride with 100g silver chlorate will produce 314.04 grams of silver telluride. The calculation involved converting the masses of the reactants to moles, applying the mole ratio from the balanced equation, and then converting back to grams using the molar mass.
To know more about zinc telluride click here:
https://brainly.com/question/31783006
#SPJ11
Considering the temperature vs. time graph below, how does the temperature at the beginning of a change of state
compare with the temperature at the end of the change?
Temperature vs. Time
140
120
100
80
60
Time (min) →
4
The temperature is always lower.
The temperature is always the same.
The temperature is usually lower.
The temperature is usually higher.
Temperature (°C)
Answers
The temperature is always the same.
How Temperature manifests in objects ?Assume we have something in solid phase. As we increase the temperature, the particles on the solid increase their kinetic energy, thus, the particles move more.
This causes that the volume of the object increases (for example when we heat up a metal and it dilates) and this keeps happening until we reach a critical point, when we are near a change of phase.
At this point the energy given is not used to increase the temperature of the object, but is used to "break" bonds in such a way that the particles are more free than before.
When all these bonds are "broken" the change of phase is completed, and in the case of the solid, we go from solid phase to liquid phase.
So, the temperature is always the same at the beginning of a change of state compare with the temperature at the end of the change
If you want to learn more about this read here ;
brainly.com/question/11804615
#SPJ1
What is the molarity of 10g of carbon in a 500L solution?
Answers
Answer:
Molarity is the number of moles in a liter of a substance.
Molarity= Mole/volume
Mass= 10g
Molar mass of Carbon=12g
To calculate the mole we use the formula: mole= mass/molar mass
Mole = 10g/12g
Mole = 0.83
Molarity= 0.83/500 =0.0017moles per liter
Why can't scientists simply use carbon-12 (12C) do determine
the age of dead carbon-based life forms?
Answers
Answer:
carbon-12 is not radioactive
Explanation:
The measurement of the age of dead carbon based life forms requires the use of a radioactive isotope hence it is often referred to as radiocarbon dating.
Carbon-12 and Carbon-14 occur together in living things.The half life of Carbon-14 is about 5670 years.
Hence, since Carbon-12 is not radioactive, it can not be used to measure the age of dead carbon based materials.
1. Justin usually blows his nose using Kleenex tissues. His snot keeps leaking through the tissue. He decided he is going to create an experiment to find out if there is something better. What is his dependent variable?
Answers
Answer:
His snot.
Explanation:
Because a dependent variable is a variable (often denoted by y ) whose value depends on that of another, and a example is test scores, those are a dependent variable. And it's his snot because his snot keeps leaking and hes going to create an experiment to see if he can solve it.
The dependent variable of Justin's experiment is the mass of the snot the tissue can hold.
What are variables?A variable can be described as an alphabet that can be used to represent an unknown number. Variable generally represents the value. A variable can be described as a quantity that can be changed according to the given mathematical problem.
The dependent variable can be defined as a variable that will depend on the value of some other number. The dependent variable is also defined as the output of a function. If the value of the dependent variable will change therefore there is a change in the value of the respective independent variable.
The independent variable of the experiment will not depend on the values of other variables and is known as the input of a function. The value of the independent variable will not affect by any values of a variable.
Learn more about Variable, here:
brainly.com/question/17344045
#SPJ2
WORTH 60 POINTS DONT PLAGIARIZE OR WILL REPORT. thanks :) You and your family are going on a trip in Europe. Calculate the speed in the following picture. Show your work and include units. Include 4-5 sentences explaining.

Answers
Answer:80
Explanation:
It would be 80 kilometers an hour. All you have to do is divide the total distance traveled which in this case is 240 kilometers, by the number of hours driven which is 3 hours.
240km/3 hours = 80km/hour
To find the answer you divide 240 by 3 in order to find how far you go in an hour.
A car travels with a force of 10,000N and has a mass of 500 kg. What is the acceleration of the car?
Answers
Explanation:
we have,
force(f)=10,000N
mass(m)=500kg
now,
acceleration=f×m
=10,000×500
=5000000m/s²
The particles in.......... can be separated from
heterogeneous mixtures by passing the mixture through a filter.
-suspension
-solution
-colloid
-pure substance
Answers
The particles in suspension can be separated from heterogenous mixtures by passing the mixture through a filter. Option 1.
Particles in a suspensionThe particles in suspension can be separated from heterogeneous mixtures by passing the mixture through a filter.
A suspension is a heterogeneous mixture in which the particles are large enough to settle out over time and can be separated by physical means such as filtration.
Other options such as solution, colloid, and pure substances cannot be separated using a filter.
More on suspensions can be found here: https://brainly.com/question/17650174
#SPJ1
The volume of a gas sample is 22.4 liters at STP. The density of the gas is 1.34 grams per liter.
What is the mass
of the gas sample, expressed to the correct number of significant figures?
Answers
Answer:
The mass of gas is 30.02 g.
Explanation:
Given data:
Volume of gas = 22.4 L
Density of gas = 1.34 g/L
Mass of gas = ?
Solution:
The given problem will be solved through density formula.
Density is equal to the mass of substance divided by its volume.
Units:
SI unit of density is Kg/m3.
Other units are given below,
g/cm3, g/mL , kg/L
Formula:
D=m/v
Now we will put the values in formula.
1.34 g/L = m /22.4 L
m = 1.34 g/L × 22.4 L
m = 30.02 g
The mass of gas is 30.02 g.
How many moles of LINO3 are
equivalent to 525 g LINO3?
(LINO3 = 68.95 g/mol)
[?] mol LINO3
Answers
Answer:
Explanation:
number of moles = mass / molar mass
= 525 / 7+14+(3*14)
= 525 / 63
= 8.33 mol
Chemistry help needed. Please help. Need it by Sunday. Please help!
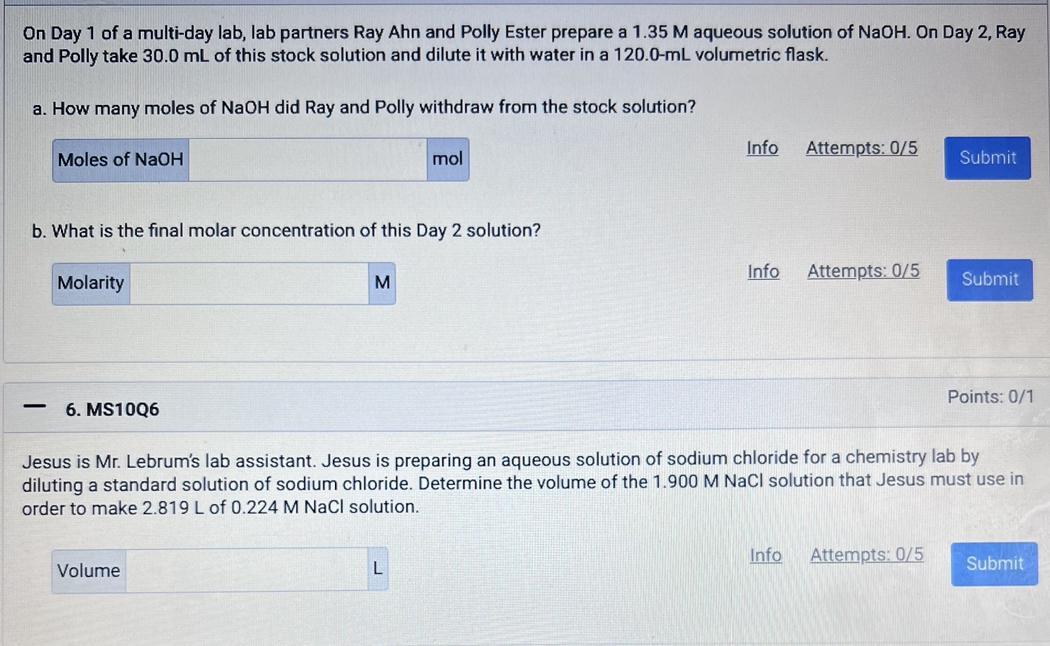
Answers
A. The mole of NaOH withdrawn from the solution is 0.0405 mole
B. The final molar concentration of the solution is 0.3375 M
C. The volume of the 1.9 M NaCl needed is 0.332 L
A. How do i determine the mole withdrawn?The mole of NaOH withdrawn from the solution can be obtained as follow:
Molarity of stock solution = 1.35 MVolume of stock solution = 30 mL = 30 / 1000 = 0.03 LNumber of mole of NaOH =?Number of mole = molarity × volume
Number of mole of NaOH = 1.35 × 0.03
Number of mole of NaOH = 0.0405 mole
B. How do i determine the final molar concentration of the solution?The final molar concentration of the solution can be obtained as follow:
Volume of stock solution (V₁) = 30 mL Molar concentration of stock solution (M₁) = 1.35 MVolume of final solution (V₂) = 120 mL Molar concentration of final solution (M₂) =?M₁V₁ = M₂V₂
1.35 × 30 = M₂ × 120
40.5 = M₂ × 120
Divide both side by 120
M₂ = 40.5 / 120
M₂ = 0.3375 M
Thus, the final molar concentration of the solution is 0.3375 M
C. How do i determine the volume of NaCl needed?The volume of the 1.9 M NaCl solution needed can be obtained as folllow:
Molarity of stock solution (M₁) = 1.9 MVolume of diluted solution (V₂) = 2.819 L Molarity of diluted solution (M₂) = 0.224 MVolume of stock solution needed (V₁) =?M₁V₁ = M₂V₂
1.9 × V₁ = 0.224 × 2.819
Divide bioth sides by 4.67
V₁ = (0.224 × 2.819) / 1.9
V₁ = 0.332 L
Thus, we can conclude that the volume of the 1.9 M NaCl solution needed is 0.332 L
Learn more about volume:
https://brainly.com/question/24159217
#SPJ1
the process that breaks down rocks
Answers
where did the atoms that make up a newborn baby originate
Answers
The atoms that make up a newborn baby originated from various sources. Primarily, these atoms were forged inside stars through nucleosynthesis, where hydrogen and helium fused to form heavier elements.
The birth and death of multiple generations of stars over billions of years contributed to the creation of these atoms. Additionally, some atoms may have been produced during cosmic events such as supernovae or stellar collisions. Ultimately, these atoms were dispersed into space and later incorporated into the material that formed Earth, including the molecules necessary for life. The atoms comprising a newborn baby have a fascinating cosmic origin. The fundamental elements, such as hydrogen and helium, were formed shortly after the Big Bang. However, the heavier elements necessary for life, such as carbon, oxygen, nitrogen, and calcium, were produced through nucleosynthesis within stars. As stars reach the end of their lifecycle, they undergo nuclear fusion processes, where immense temperatures and pressures cause lighter elements to merge and form heavier ones. Elements up to iron are typically synthesized through stellar nucleosynthesis. During a supernova explosion, massive stars release tremendous energy and scatter these newly formed atoms into space. Supernovae are critical in dispersing heavier elements throughout the universe. These atoms then mix with interstellar gas and dust, eventually becoming part of molecular clouds, which are regions of space where new stars and planetary systems form. Over time, gravitational forces cause these clouds to collapse, leading to the formation of new stars and their associated planetary systems. The birth and death of multiple generations of stars have played a crucial role in the formation of the atoms present in a newborn baby. Each stellar generation enriches the interstellar medium with heavier elements, which are incorporated into subsequent generations of stars and their planetary systems. Furthermore, cosmic events like stellar collisions can also contribute to the production of heavy elements. The atoms from these processes eventually become part of the material that forms planets like Earth. On Earth, the atoms essential for life come together in various compounds, including water, amino acids, and nucleotides, which are the building blocks of DNA and proteins. These molecules are synthesized through chemical reactions that occur in the oceans, atmosphere, and even within living organisms themselves. Eventually, these complex molecules combine to form cells, and through a process of growth and development, they give rise to a newborn baby. In summary, the atoms that make up a newborn baby have their origins in the nucleosynthesis processes occurring within stars. The birth and death of stars, supernova explosions, stellar collisions, and the subsequent formation of planets have all contributed to the creation and dispersion of these atoms throughout the universe. Through complex chemical reactions and biological processes on Earth, these atoms come together to form the molecules necessary for life and ultimately give rise to a newborn baby
Learn more about Big Bang here: brainly.com/question/17209127
#SPJ11
.
The earth is slightly tilted on its axis. How much is it tilted?
23.5 degrees
28 degrees
20.5 degrees
25 degrees
Answers
Cu2(s)+O2(g)=Cu2O(g)+SO2(g)
please help urgent will give brainiest
Answers
Answer:
2 Cu2S + 3 O2 = 2 Cu2O + 2 SO2
Explanation:
2 Cu2S + 3 O2 = 2 Cu2O + 2 SO2
Which best sequences the organization of the Universe from LARGEST to SMALLEST? *
Asteroids, Moons, Planets, Stars, Star Systems, Galaxies, Galaxy Clusters, Universe
Galaxy Clusters, Galaxies, Stars, Planets, Moons, Comets
Universe, Stars, Planets, Asteroids
Universe, Galaxies Clusters, Galaxies, Star Systems, Stars, Planets, Moons, Asteroids
Answers
Universe, Stars, Planets, Asteroids
Answer:
Universe, Stars, Planets, Asteroids
Explanation:
a 250 gram sample of water at the boiling point had 35.0 kj of heat added. how many grams of water were vaporized? heat of vaporization for water is 40.6 kj/mole.
Answers
The required mass of water vaporized is 15.5 grams, from a 250 gram sample of water at the boiling point had 35.0 kj
Given: Mass of water (m) = 250 gHeat added (q) = 35.0 kJHeat of vaporization (ΔHvap) = 40.6 kJ/mole
To find:Mass of water vaporized (x) Formula:q = ΔHvap × nx = (q / ΔHvap) × nMass = moles × molar mass
We know that molar mass of water (H2O) = 18 g/molMoles of water vaporized (n) = (35.0 kJ / 40.6 kJ/mol) = 0.861 mol
Therefore,Mass of water vaporized (x) = 0.861 mol × 18 g/mol= 15.5
Detailed Solution: According to the given statement,250g of water was taken at its boiling point and 35.0 kJ of heat was added to it, we need to find how many grams of water were vaporized. To solve this question, first, we need to know the heat of vaporization for water, which is 40.6 kJ/mole. It means to vaporize 1 mole of water, 40.6 kJ of heat is required.
Mass of water (m) = 250 g Heat added (q) = 35.0 kJHeat of vaporization (ΔHvap) = 40.6 kJ/molen = q / ΔHvapn = (35.0 kJ / 40.6 kJ/mol) = 0.861 molMoles of water vaporized (n) = 0.861 mol
Therefore, Mass of water vaporized (x) = 0.861 mol × 18 g/mol= 15.5 g Hence, the required mass of water vaporized is 15.5 grams.
To learn more about mass visit;
https://brainly.com/question/11954533
#SPJ11
he scientist had a 35% saline solution that he mixed with 10 milliliters of a 75% saline solution to get a 40% saline solution. How many milliliters of the 35% solution were used? a) 30 milliliters b) 40 milliliters c) 50 milliliters d) 60 milliliters e) 70 milliliters
Answers
The answer is (e) 70 milliliters.
Let's assume that x milliliters of the 35% saline solution were used.
The total amount of saline in the solution can be calculated as follows:
Saline in 35% solution = 0.35 * x
Saline in 75% solution = 0.75 * 10 (since 10 milliliters of the 75% solution were used)
The total amount of saline in the resulting 40% solution can be calculated as:
Saline in 40% solution = 0.40 * (x + 10)
Since the saline is being mixed, the total saline in the resulting solution is equal to the sum of the saline in the individual solutions:
0.35 * x + 0.75 * 10 = 0.40 * (x + 10)
Simplifying the equation:
0.35x + 7.5 = 0.40x + 4
Subtracting 0.35x and 4 from both sides:
7.5 - 4 = 0.40x - 0.35x
3.5 = 0.05x
Dividing both sides by 0.05:
x = 3.5 / 0.05
x = 70
Therefore, 70 milliliters of the 35% saline solution were used. The answer is (e) 70 milliliters.
For more details regarding the saline solution, visit:
https://brainly.com/question/24498665
#SPJ4
Which is the correct short-hand notation for the cell that you will study in this experiment?Mg | Mg2+ || Hg2+ | HgMg | Mg2+ || Cu2+ | CuCu | Cu2+ || Mg2+ | MgHg | Hg2+ || Mg2+ | Mg
Answers
The correct short-hand notation for the cell that will be studied in this experiment depends on the specific experimental setup and the desired electrochemical reaction. In the first given notation, Mg is the anode and Hg2+ is the cathode.
In the second given notation, Mg is still the anode but Cu2+ is the cathode. In the third given notation, Cu is the anode and Mg2+ is the cathode. In the fourth given notation, Hg is the anode and Mg2+ is the cathode. To determine the correct notation, specific experimental conditions must be considered, including the type and concentration of electrolyte solutions, temperature, and the desired direction of electron flow. It is important to note that the short-hand notation is a simplified representation of the electrochemical cell and may not capture all aspects of the reaction. In general, the short-hand notation is written with the anode on the left and the cathode on the right, separated by double vertical bars indicating a salt bridge or other ion-permeable barrier. The electrode materials and their respective ions are written as half-reactions with the anode on the left and the cathode on the right, separated by single vertical bars.
To know more about the electrochemical reaction.
https://brainly.com/question/31604301
#SPJ11
Many animals influence and contribute to ecosystem services. As pollinators, how do butterflies ultimately contribute to direct ecosystem services?
Answers
Answer:
yEs
Explanation:
The chemical formula for naphthalene is C10H8. It’s used to make mothballs and pesticides.
In 4C10H8, the coefficient is , the subscript of carbon is , and the subscript of hydrogen is .
Answers
In \(4C_{10}H_8\), the coefficient is 4, the subscript of carbon is 10, and the subscript of hydrogen is 8.
Coefficients of chemical formulasChemical formulas can be empirical or molecular. Empirical formulas have the lowest possible whole-number ratio of atoms that make up substances. Molecular formulas, on the other hand, could have whole-number ratios that could be in multiples.
Thus, each atom in chemical formulas has its respective subscripts which indicate the amount of the atom present in the formula. In chemical equations, the coefficients are the number written before chemical formulas.
Thus, in \(4C_{10}H_8\), the coefficient is 4, the subscript of carbon is 10, and the subscript of hydrogen is 8.
More on chemical formulas can be found here: https://brainly.com/question/29031056
#SPJ1
write a net ionic equation for the reaction that occurs when aqueous solutions of ammonia and hydroiodic acid are combined.
Answers
The reaction between aqueous solutions of ammonia (NH3) and hydroiodic acid (HI) can be represented by the following balanced chemical equation:
NH3(aq) + HI(aq) → NH4I(aq)
To write the net ionic equation, we need to break down the aqueous compounds into their respective ions, considering only the species that participate in the chemical reaction. In this case, ammonia (NH3) does not ionize significantly in water, but hydroiodic acid (HI) dissociates completely to form ions:
NH3(aq) + H+(aq) + I-(aq) → NH4+(aq) + I-(aq)
The net ionic equation can be written by eliminating the spectator ions
NH3(aq) + H+(aq) → NH4+(aq)
Therefore, the net ionic equation for the reaction between aqueous solutions of ammonia and hydroiodic acid is:
NH3(aq) + H+(aq) → NH4+(aq).
Learn more about ionic equation here ;
https://brainly.com/question/13887096
#SPJ11
Ethanol fuel mixtures have "E" numbers that indicate the percentage of ethanol in the mixture by volume. For example, E10 is a mixture of 10% ethanol and 90% gasoline. How much E7 should be mixed with 3000 gal of E10 to make an E9 mixture? Part: 0 / 4 Part 1 of 4 Let x represent the amount of a mixture (in gal) containing 319. ethanol. 3000 gal is the amount of E10 mixture containing 10% ethanol. Therefore, is the amount of the resulting E9 mixture containing 906 ethanol
Answers
To make an E9 mixture 8657.14 gal of E7 should be mixed with 3000 gal of E10
Given to us is the amount of ethanol in the E10 mixture is 10% of 3000 gallons:
Ethanol in E10 = 10% × 3000 gal = 0.10 × 3000 gal = 300 gal
To solve this problem, we can set up an equation based on the amount of ethanol in each mixture.
Let x represent the amount of E7 mixture (in gallons) that needs to be added to the E10 mixture to obtain the desired E9 mixture.
The amount of ethanol in the E7 mixture is 7% of x gallons:
Ethanol in E7 = 7% × gal = 0.07 × gal
The resulting E9 mixture will contain 9% ethanol of the total volume of 3000 + x gallons:
Ethanol in E9 = 9% × (3000 + x) gal = 0.09 × (3000 + x) gal
According to the problem, the resulting E9 mixture contains 906 gallons of ethanol:
Ethanol in E9 = 906 gal
Now we can set up the equation:
Ethanol in E10 + Ethanol in E7 = Ethanol in E9
300 gal + 0.07x gal = 906 gal
Subtracting 300 gal from both sides:
0.07x gal = 606 gal
Dividing both sides by 0.07:
x = 606 gal / 0.07
x = 8657.14
Therefore, approximately 8657.14 gallons of E7 mixture should be mixed with 3000 gallons of E10 to make an E9 mixture.
Learn more about ethanol mixtures here:
https://brainly.com/question/28954299
#SPJ4
Complete question: Ethanol fuel mixtures have "E" numbers that indicate the percentage of ethanol in the mixture by volume. For example, E10 is a mixture of 10% ethanol and 90% gasoline. How much E7 should be mixed with 3000 gal of E10 to make an E9 mixture?
what term should be used to describe the presence of a higher concentration of a salt within a fluid than the volume is able to dissolve to maintain equilibrium?
Answers
In fact, sodium, potassium, or ammonium sulfates enhance ligand-protein interactions in HIC while also stabilizing protein structure. As a result, the most prevalent ions are (NH4)2SO4, Na2SO4, NaCl, KCl, and CH3COONH4. Figure 1 depicts how various salts can influence selectivity. The highest resolution of four standard proteins was achieved by starting with 1.7 M ammonium sulfate.
Because each salt has a different capacity to encourage hydrophobic interactions, selecting a salt for an HIC separation can be a trial and error process. When the concentration of a salt rises, the quantity of protein bound increases almost linearly up to a certain salt concentration and then exponentially at greater concentrations.When compared to other salts, ammonium sulfate often provides the greatest clarity at a particular concentration and can be used at ratios up to 2 M.
When using sodium chloride, concentrations of up to 3 M are typically needed.
Although sodium sulfate is an excellent salting-out substance, issues with protein solubility may preclude its use at high amounts.
Working with ammonium sulfate at pH levels above 8.0 is not advised.
An experiment was conducted to estimate the effect of smoking on the blood pressure of a group of 37 cigarette smokers. The difference for each participant was obtained by taking the difference in the blood pressure readings at the beginning of the experiment and again five years later. The sample mean increase, measured in millimetres of mercury, was x = 9.1. The sample standard deviation was s = 5.5. Estimate the mean increase in blood pressure that one would expect for cigarette smokers over the time span indicated by the experiment. Find the 95% margin of error. (Round your answer to two decimal places
Answers
The 95% margin of error for the mean increase in blood pressure that one would expect for cigarette smokers over the time span indicated by the experiment is ±1.98 (rounded off to two decimal places).
The mean increase in blood pressure that one would expect for cigarette smokers over the time span indicated by the experiment can be estimated by using the formula;μ = x ± z(\(a^{2}\)) * σ/√n
Where;μ is the population mean increase.x is the sample mean increase.z(\(a^{2}\)) is the z-scoreα is the level of significanceσ is the population standard deviationn is the sample size.
Substituting the given values into the formula;μ = 9.1 ± 1.96 * 5.5/√37= 9.1 ± 1.98
The mean increase in blood pressure that one would expect for cigarette smokers over the time span indicated by the experiment lies between 7.12 to 11.08.
Hence, the estimated mean increase is between 7.12 to 11.08 millimeters of mercury.
The 95% margin of error can be calculated using the formula;
Margin of error (E) = z(\(a^{2}\)) * σ/√n
Margin of error (E) = 1.96 * 5.5/√37
Margin of error (E) = 1.98 (approximated to two decimal places).
To know more about standard deviation visit :
https://brainly.com/question/31850069
#SPJ11
What is a good description of bacterial reproduction? (1 point)
Responses
rapid reproduction through binary fission
slow reproduction through sexual reproduction
fast reproduction through sexual reproduction
slow reproduction through binary fission
Answers
A good description of bacterial reproduction is rapid reproduction through binary fission. That is option A.
What is binary fission?Binary fission is defined as the type of asexual reproduction whereby an organism multiplies to form new organisms through the separation of the body into two new bodies.
The process of binary fission include the following:
The organism such as bacteria, duplicates its genetic material( deoxyribonucleic acid).It then divides into two parts (cytokinesis),After each division, the new organism receive one copy of DNA. each.Under ideal conditions some bacterial species may divide every 10–15 minutes—a doubling of the population at these time intervals.
Therefore, the good description of bacterial reproduction is rapid reproduction through binary fission.
Learn more about reproduction here:
https://brainly.com/question/29762377
#SPJ1
calculate the percent recovery from the acetanilide recrystallization. show calculation with units and correct significant digits. was this recrystallization successful at purifying the acetanilide? use your written observations of the physical appearance of the acetanilide (before and after recrystallization) and the melting point range of the purified acetanilide as evidence.
Answers
The percent recovery of acetanilide recrystallization is: 75.5%,
the physical appearance of the purified acetanilide was: white, fine-grained crystals
and the melting point range of the purified acetanilide was: 114.6-116.9 °C
Explanation:
The percent recovery of acetanilide recrystallization is as follows: Initial weight of acetanilide = 2.245 g
Weight of filter paper = 0.343 g
Weight of filter paper + purified acetanilide = 2.633 g
Weight of purified acetanilide = (2.633 - 0.343) g = 2.290 g
Percent recovery = (Weight of purified acetanilide / Initial weight of acetanilide) × 100= (2.290 / 3.032) × 100 = 75.5%
Since the percent recovery is greater than 60%, we can say that the acetanilide recrystallization was successful at purifying the acetanilide.
The physical appearance of the purified acetanilide was white, fine-grained crystals, and the melting point range of the purified acetanilide was 114.6-116.9 °C. Both the physical appearance and melting point range confirm that the acetanilide was purified through the recrystallization process.
To know more about recrystallization refer here:
https://brainly.com/question/15703840#
#SPJ11
How many times bigger is the radius of a helium atom than the radius of an alpha particle.
Answers
The radius of a helium atom is 4.8 times the radius of an alpha particle.
What is alpha particle?
Alpha particles, also called alpha rays and alpha radiation, have a structure similar to that of the helium-4 nucleus, consisting of two protons and two neutrons bonded together.
What is helium?
Helium has been used as an inert gas environment for welding metals such as aluminum. It is also used for rocket propulsion.
The radius of a helium atom is 4.8 times the radius of an alpha particle. The diameter of the nucleus of the helium atom, an alpha particle, was also measured more accurately than ever before. The results only show a size of 1.6782 femtometers, making him 4.8 times more accurate than previous measurements.
Therefore, The radius of a helium atom is 4.8 times the radius of an alpha particle.
To know more about helium and alpha particle, visit:
https://brainly.com/question/2288334
#SPJ4
Which answer tells the reason the earth’s climate is getting warmer?
a. Large glaciers are melting in Antarctica.
b. The earth is moving closer to the sun.
c. Driving cars gives off gases that trap heat in the atmosphere.
d. Too many animals are becoming extinct.
if right i give brain 5 star and thx
Answers
Answer:
The correct answer is C) Driving cars gives off gases that trap heat in the atmosphere.
Answer:
the answer is c
Explanation: