A hiker inhales 598 ml of air. if the final volume of air in the lungs is 612 ml, at a body temperature of 37 degrees celsius, what was the initial temperature of the air in degrees celsius? explain.
Answers
The initial temperature of the air in degree Celsius was approximately 33.6°C.
When the hiker inhales air, the air undergoes a temperature change from the initial temperature to the body temperature, and a volume change due to the expansion of the lungs.
Using the ideal gas law, we can relate the initial and final volumes and temperatures of the air.
PV = nRT
Assuming the pressure is constant, we can rearrange the equation to:
(V₁/T₁) = (V₂/T₂)
where V1 is the initial volume of air, T₁ is the initial temperature, V₂ is the final volume of air, and T₂ is the final temperature (body temperature, 37°C).
We can substitute the given values and solve for T₁:
(V₁/T₁) = (V₂/T₂)
(T₁/V₁) = (T₂/V₂)
T₁= (T2 × V₁ / V₂
T₁ = (310.15 K × 0.598 L) / 0.612 L
T₁≈ 303.5 K
Converting to degrees Celsius, we get:
T₁ ≈ 30.5°C
To know more about ideal gas law, refer here:
https://brainly.com/question/30458409#
#SPJ11
Related Questions
What is metal ? Where does it coke from ?
Answers
Answer:
Most pure metals, like aluminium, silver and copper, come from the Earth's crust. They are found in ores – solid materials called minerals, usually occurring in rock, from which the pure metal has to be extracted. The properties of pure metals can be improved by mixing them with other metals to make alloys.a metal is an element that readily forms positive ions (cations) and has metallic bonds
coke is a strong stimulant made from coca leaves
Bài 1. Có 30g dung dịch NaCl 20%. Tính nồng độ % dung dịch thu được khi
a. Pha thêm 20g H2O
b. Cô đặc dung dịch để chỉ còn 25g.
Answers
Answer:
Bài 1. Có 30g dung dịch NaCl 20%. Tính nồng độ % dung dịch thu được khi
a. Pha thêm 20g H2O
b. Cô đặc dung dịch để chỉ còn 25g.
Use the web to determine the safety of the spring water sample. explain if a particular web component of the water could be hazardous for consumption.
Answers
The safety of the spring water sample is said to be that in an untreated state, it is said to be undrinkable.
What is spring water?The EPA is one that tells that the spring water is seen as a kind of any water that is said to have its origin from any kind of underground aquifer and is said to be taken as it flows naturally to the earth's surface or through the use of a borehole that lunch into the underground water source.
Note that studies shows that when a spring water is said to be examined, the different parameters of health safety were said to be analyzed by the use of laboratory tests and the outcome of the study reveals that water is not good for drinking because it has higher concentrations of ammonium ion as well as others.
Hence, The safety of the spring water sample is said to be that in an untreated state, it is said to be undrinkable.
Learn more about spring water from
https://brainly.com/question/2375655
#SPJ1
Chemical Reactions - Problems
4 NH, +5 O, → 4 NO+6H_O
Which of the following are the reactants in the reaction above?
NH,
NO
I
H₂O
Submit v
IT
Answers
What makes a person scientifically literate? In three to five sentences, explain your answer.
Answers
It means that a person has the ability to describe, explain, and predict natural phenomena. Scientific literacy entails being able to read with understanding articles about science in the popular press and to engage in social conversation about the validity of the conclusions. Scientific literacy implies that a person can identify scientific issues underlying national and local decisions and express positions that are scientifically and technologically informed. A literate citizen should be able to evaluate the quality of scientific information on the basis of its source and the methods used to generate it. Scientific literacy also implies the capacity to pose and evaluate arguments based on evidence and to apply conclusions from such arguments appropriately.
hope this helps and sorry if this is wrong
which indicator has to be used in order to get an emerald green color at a pH of 9?
a) universal indicator
b) methyl orange indicator
c) thymol blue indicator
d) cyanidin
Answers
Answer:
D) Cyanidin indicator
Explanation:
Cyanidin indicator has to be used in order to get an emerald green color at a pH of 9.
What is an indicator?Indicators are substances that change colour when they are added to acidic or alkaline solutions.
In nature, cyanidin is a reddish-purple (magenta) pigment. It is the major pigment in berries [4] and other red-coloured vegetables such as red sweet potato and purple corn. It appears as a blue-reddish or purple pigment in the plant.
Cyanidin indicator has to be used in order to get an emerald green colour at a pH of 9.
Learn more about indicators here:
https://brainly.com/question/488857
#SPJ2
what is saturated solution?12
Answers
Answer:
A solution in which the maximum amount of solvent has been dissolved
Explanation:
For example, 36g of salt in 100g of water.
The molar heat of vaporization for liquid water is 40.6 kJ/mole.
How much energy is required to change 5.2 g of liquid water to steam if the water is already at 100oC?
Answers
So, if the water is already at 100oC, it would take 11,725 J of energy to convert 5.2 g of liquid water to steam.
For liquid water, the molar heat of vaporization is 40.6 kJ/mol4. If the water is already at 100°C, we can use the following formula to determine the amount of energy needed to convert 5.2 g of liquid water to steam:
Q = mL
What exactly is vaporization heat?
The quantity of enthalpy (heat energy) necessary to convert a liquid substance into a gas or vapour is known as the heat of vaporization1. Joules per mole (J/mole) or, in some cases, calories are used to measure it (C). Enthalpy of vaporization is another name for the heat of vaporization
m is the mass of water being vaporized (in grammes), Q is the needed energy, and L is the molar heat of vaporization (in J/mole).
https://brainly.com/question/29401184
To begin, we must change 5.2 g of water into moles:
18.015 g/mole / 5.2 g = 0.288 mole
Then, we can determine Q:
Q is equal to 0.28 mole, 40.6 kJ/mole, and 1000 J/kJ.
Q = 11,725 J
To know more about vaporization heat visit:
brainly.com/question/29401184
#SPJ1
18. In order to make one molecule of glucose, how many carbon dioxide, ATPs, and NADPH are required?
Answers
To produce one molecule of glucose, 6 molecules of carbon dioxide (\(CO_{2}\)), 18 molecules of adenosine triphosphate (ATP), and 12 molecules of nicotinamide adenine dinucleotide phosphate (NADPH) are required.
Glucose, a six-carbon sugar, is synthesized through the process of photosynthesis in plants. It involves the Calvin cycle, which incorporates carbon dioxide, ATP, and NADPH to produce glucose. For each molecule of glucose formed, 6 molecules of carbon dioxide are required.
The energy needed for glucose synthesis is provided by ATP, which is an energy-rich molecule. In the Calvin cycle, the synthesis of one glucose molecule requires 18 molecules of ATP.
NADPH, a coenzyme involved in energy transfer reactions, is required for the reduction of carbon dioxide during the Calvin cycle. In the process, 12 molecules of NADPH are utilized to produce one molecule of glucose. These components play crucial roles in capturing and storing energy, as well as providing carbon atoms for the formation of glucose, which serves as a vital energy source for organisms.
Learn more about Calvin cycle here:
https://brainly.com/question/26846190
#SPJ11
when 51.8 and 67.5 are multiplied, the product has ____ significant figures, and when 51.8 and 67.5 are added, the sum has ____ significant figures.
Answers
when 51.8 and 67.5 are multiplied, the product has three significant figures, and when 51.8 and 67.5 are added, the sum has three significant figures.
The significant figures in multiplying 51.8 and 67.5:
51.8 × 67.5 = 3496.5 = 3.50 × 10^3
Now according to the rules of significant figures
All non-zero numbers and trailing zeros to the right of the decimal are significant. But 10 and power of ten like in this case its 3 are not significant. So the product has three significant figures.
The significant figures in adding 51.8 and 67.5:
51.8 + 67.5 = 119.5
Now, according to the rules of significant figures, all non-zero digits are significant figures. So the sum has 4 significant figures.
If you need to learn more about significant figures, click here
https://brainly.com/question/11905420?referrer=searchResults
#SPJ4
Why could we see different colors in kaleidoscope lane
Answers
The different colors in Kaleidoscope Lane can be attributed to a mix of colorful objects, reflective surfaces, diverse light sources, and the interaction of colors, creating a visually captivating environment similar to the patterns seen in a kaleidoscope.
The different colors in Kaleidoscope Lane can be seen due to the combination of the following factors:
1. Colorful objects: The lane might be decorated with various colorful items like street art, murals, or decorations, which contribute to the vibrant colors seen in the area.
2. Reflection: A kaleidoscope is an optical instrument that uses mirrors to reflect light and create symmetrical patterns. In Kaleidoscope Lane, reflective surfaces or mirrored installations might be placed to bounce light off and create colorful patterns similar to those seen in a kaleidoscope.
3. Light sources: Different colored lights or filters might be installed in the lane to illuminate the surroundings, which results in a variety of colors being seen.
4. Interaction of colors: As light bounces off multiple surfaces and interacts with various colored objects in the lane, the colors blend and create new combinations, further contributing to the visual spectacle.
learn more about Kaleidoscope Refer: brainly.com/question/24359686
#SPJ11
it is on SWRO plant with a capacity of 50000m3/day the tds of the feed is 41690ppm implying a chloride ion level of around 23000ppm the temperature of the feed is around 18°C in March and 27°C in September the reject has a tds of 64500ppm . the pressure is 70 bar, that plant started to produce water in June 2003 and corrosion problem appeared already few months of service, two type of corrosion could be established, one being crevice corrosion in 11/2" high pressure connector underneath victauling coupling example the same type of problem that have been corrosion in 316L and 317L high pressure piping seven out of 700 such connector were reported to have suffered this type crevice corrosion after 4 months only, provide the remedy to end the problem
Answers
To address the crevice corrosion issue in the high-pressure connectors and piping of the SWRO plant, several remedies can be considered, A SWRO (Sea Water Reverse Osmosis) plant is a water desalination facility that uses reverse osmosis technology to treat seawater or brackish water and produce freshwater
Material Selection: Evaluate the material compatibility with the operating conditions, especially the chloride ion concentration and temperature. Consider using corrosion-resistant alloys such as duplex stainless steel (e.g., 2205) or super duplex stainless steel (e.g., 2507) that have better resistance to chloride-induced corrosion compared to 316L or 317L stainless steel.
Surface Treatment: Apply appropriate surface treatments to enhance corrosion resistance. Passivation or pickling can remove surface contaminants and create a protective oxide layer on the metal surface, reducing the susceptibility to corrosion.
Design Modifications: Evaluate the design of the connectors and piping to minimize crevices and stagnant areas where corrosion can occur. Smooth transitions, avoiding sharp angles or crevices, can help promote better fluid flow and prevent the accumulation of corrosive substances.
Cathodic Protection: Implement cathodic protection methods, such as impressed current or sacrificial anodes, to protect the connectors and piping from corrosion. This technique involves introducing a more easily corroded metal (anode) to the system, which sacrifices itself to protect the connected metal (cathode) from corrosion.
Monitoring and Maintenance: Regularly monitor the corrosion levels and condition of the connectors and piping. Implement a maintenance program that includes periodic inspections, cleaning, and repairs, if necessary, to prevent corrosion from progressing.
It is important to consult with corrosion experts and engineers who specialize in SWRO plant operations to assess the specific conditions, perform material testing, and provide tailored solutions to mitigate the crevice corrosion problem effectively.
To know more about SWRO (Sea Water Reverse Osmosis), click here, https://brainly.com/question/31556553
#SPJ11
Hot air balloons use a flame to heat gas. when the flame turns on, the temperature increases. what happens to the volume of the gas?
Answers
When the flame turns on and heats the gas inside the hot air balloon, the volume of the gas increases.
This is because as the temperature of a gas increases, the molecules in the gas move faster and collide more frequently, creating a greater pressure. This increase in pressure causes the gas to expand, which results in an increase in volume. Therefore, when the flame turns on and heats the gas inside the hot air balloon, the gas molecules move faster, collide more frequently, and create a greater pressure, causing the gas to expand and the volume to increase.
This phenomenon can be explained using the Gas Laws, specifically Charles's Law. Charles's Law states that the volume of a gas is directly proportional to its temperature, as long as the pressure and the amount of gas remain constant.
learn more about Gas Laws
https://brainly.com/question/27870704
#SPJ11
which compound contains only covalent bonds? group of answer choices ca3(po4)2 no2 nh4oh nacl
Answers
The compound that contains only covalent bonds among the given choices is NO₂ (nitrogen dioxide).
Covalent bonds are formed when atoms share electrons, typically between non-metal atoms. In NO₂, nitrogen (N) and oxygen (O) are both nonmetals, and they share electrons to form covalent bonds. On the other hand, the other compounds listed contain both ionic and covalent bonds:
Ca₃(PO₄)₂ (calcium phosphate) contains both ionic and covalent bonds. The calcium cation (Ca²⁺) forms ionic bonds with the phosphate anions (PO₄³⁻), while the phosphate group itself contains covalent bonds.
NH₄OH (ammonium hydroxide) contains both ionic and covalent bonds. The ammonium cation (NH₄⁺) forms ionic bonds with the hydroxide anion (OH⁻), while the covalent bond exists within the ammonium group.
NaCl (sodium chloride) contains ionic bonds. Sodium (Na) is a metal and donates an electron to chlorine (Cl), a nonmetal, resulting in the formation of an ionic compound.
Therefore, the compound NO₂ is the only one among the given choices that contains only covalent bonds.
Learn more about covalent bonds here:
https://brainly.com/question/30031358
#SPJ11
Which two types of clouds produce precipitation (rain)?
Answers
Answer:
nimbostratus (cumulonimbus) and stratus
Explanation:
Which of these statements is false?
Select one:
a. Electrons have a negative charge
b. Electrons have a mass of 1 amu
c. The nucleus of an atom is positively charged
d. The neutron is found in the nucleus of an atom
Answers
Answer:
b. Electrons have a mass of 1 amu
Explanation:
Mass of electron is 0.00055amu.
Charge= - 1.6×10^−19
By definition, the statement b. is false: Electrons haven't a mass of 1 amu. Electrons have a mass of 0.0005 amu.
All atoms are made up of subatomic particles: protons and neutrons, which are part of their nucleus, and electrons, which revolve around them.
Protons are positively charged, neutrons are neutrally charged, and electrons are negatively charged. Protons and neutrons have roughly the same mass, about 1.674×10⁻²⁴ g, which represents 1 atomic mass unit or amu. An electron has a mass of 9.11×10⁻²⁸ g, which represents 0.0005 amu.
So, the atomic nucleus is the small central part of the atom, with a positive electrical charge and in which most of the mass of the atom is concentrated.
In a neutral atom, the number of electrons is equal to the number of protons, and since they have a negative electrical charge, they compensate.
Then, the statement b. is false: Electrons haven't a mass of 1 amu. Electrons have a mass of 0.0005 amu.
Learn more about an atom:
https://brainly.com/question/14247445?referrer=searchResults
https://brainly.com/question/25663043?referrer=searchResults
g Determine whether the statements below are true or false. I. The relationship between the concentrations of reactants and products of a system at equilibrium is given by the law of mass action. [ Select ] II. At equilibrium, the concentrations of the reactants and products are constant over time. [ Select ]
Answers
True is the answer to statement I, and true is the answer to statement II. The relationship between the concentrations of reactants and products of a system at equilibrium is given by the law of mass action.
In other words, the mass action law states that the rate of a chemical reaction is proportional to the concentrations of the reactants. The concentrations of the reactants and products are constant over time when the system reaches equilibrium. The rate of the forward reaction is equal to the rate of the reverse reaction at equilibrium, and there is no net change in the concentration of the reactants and products. When there is a disturbance to an equilibrium system, such as changing the temperature or pressure, the system will shift to re-establish equilibrium.
The two statements given are true, and are in line with the concept of chemical equilibrium. When a chemical reaction reaches equilibrium, the concentrations of the reactants and products no longer change. At equilibrium, the rate of the forward reaction is equal to the rate of the reverse reaction, and the equilibrium position can be changed by changing the temperature, pressure, or concentration of the reactants or products. The mass action law is a mathematical equation that relates the concentrations of the reactants and products to the rate of the chemical reaction. The equilibrium constant is derived from the mass action law and is used to predict the position of equilibrium for a chemical reaction.
To know more about equilibrium visit:
brainly.com/question/30807709
#SPJ11
Please help me with Part B and Part C!

Answers
Answer:
B.
No light
C.
Explanation:
B. Maximum in A, Minimum in B
When a colored filter is placed in the path of white light, the light passes through the filter, is of the same color as that of filter.
So, when the white light passes through the red filter, the red light pass through it.
When red light pass through the green filter, the light passes through the green filter is none, so this is dark.
C.
When the nail is placed in ordinary water, it gets rusted due to the oxygen present in water.
When nail is placed in boiling water which is covered with oil, so the rust is very small quantity as boiling water also contains some oxygen.
When the nail is placed in calcium carbonate, it absorb all the moisture of air and no rusting takes place.
So, maximum rusting in test tube A and minimum rusting is in test tube C.
If you observe a Full Moon on Jan 12th, on what date would you observe the next New Moon?
Answers
Answer:
January, 28th.
Explanation:
yeet
BRAINLIEST
Bridgette wants to conducts science experiment using copper (Cu), salt (NaCl), and vinegar (C₂H₄O₂). Of these, only copper is an element. Why is this true?
Copper is the only material consisting of a single pure substance
Copper is the only material found in nature
Copper is the only material that is a solid
Copper is the only material composed of more than one pure substance
Answers
Answer:
copper is the only material composed of a single pure substance
Explanation:
plss mark brainliest
Answer:
1st opt.
(copper is the only material consisting of a single pure substance)
Explanation:
an element is made up of only one type of atom, and copper (Cu) as you can see is a single pure atom whereas salt (NaCl) and vinegar (C2H4O2) are both made of different elements.
Hope this helps
the electronic geometry of a carbon dioxide molecule would be
Answers
The electronic geometry of a carbon dioxide molecule would be linear.Electronic geometry is the shape that occurs when lone pairs and bonding pairs of electrons on a central atom repel one another to create the most stable shape.
The valence-shell electron-pair repulsion (VSEPR) theory can be used to predict the electronic geometries of molecules. The shape of a molecule is determined by its electronic geometry, which depends on the number of lone pairs and bonding pairs on the central atom of the molecule.
The electronic geometry of carbon dioxide Carbon dioxide is a linear molecule. The CO2 molecule is made up of a carbon atom in the middle, with two oxygen atoms on either side of it. The electronic geometry of CO2 is linear.Each oxygen atom has two electron groups, one of which is a double bond between carbon and oxygen, while the other is a lone pair of electrons.
The carbon atom in the center has only two electron groups, both of which are double bonds between carbon and oxygen. As a result, the carbon dioxide molecule has a linear electronic geometry.
To know more about electronic geometry visit:
https://brainly.com/question/6329970
#SPJ11
A student measured the temperature of ice water and recorded it as 0oCelsius. He then added salt to the ice water. What do you think will happen to the temperature of the ice water?
Answers
You make a solution of a weak acid with a pH of 3.75 and the pKa is 5.42 1. Is the solution acidic or basic? 2. Calculate the [H30]. 3. Calculate the pOH 4. Calculate the [OH] 5. Calculate the pKo 6. Calculate the Kb
Answers
The solution is acidic, and the [H₃O⁺] is 1.78 x 10⁻⁴ M. The pOH is 10.25, the [OH-] is 5.62 x 10⁻¹¹ M, the pKw is 14, and the Kb is 3.16 x 10⁻⁹.
1. Since the pH is less than 7, the solution is acidic.
2. To calculate the [H₃O⁺], use the formula pH = -log[H₃O⁺]. Rearrange to [H₃O⁺] = \(10^-^p^H\)= \(10^-^3^.^7^5\) = 1.78 x 10⁻⁴ M.
3. Calculate the pOH by subtracting the pH from 14: pOH = 14 - 3.75 = 10.25.
4. To calculate the [OH⁻], use the formula pOH = -log[OH⁻]. Rearrange to [OH-] = \(10^-^p^O^H\) = \(10^-^1^0^.^2^5\) = 5.62 x 10⁻¹¹ M.
5. The pKw (ion product constant of water) is always 14 at 25°C.
6. Calculate the Kb using the relationship Ka * Kb = Kw. First, convert pKa to Ka: Ka = \(10^-^p^K^_a\) = \(10^-^5^.^4^2\) . Then, Kb = Kw / Ka = 10⁻¹⁴ / \(10^-^5^.^4^2\) = 3.16 x 10⁻⁹.
To know more about ion product constant click on below link:
https://brainly.com/question/9876097#
#SPJ11
Actual answer please

Answers
a student uses a glue stick with an area of 4 cm3, putting
a pressure of 0.5 N/cm2 on her book. Calculate the force
she puts on the glue stick.
Answers
Answer:
So F=2N
Hope this helps.
Explanation:
P= F/A, where P is pressure, F is force, A is area.
So
P=F/A
0.5N/cm2 = F/4cm2 <--(do cross
2N=F multiplication,
4×0.5)
( And pls check on the unit of area u wrote, it should be (4cm2), not (4cm3) Unit of area is cm2.)
Which of the following explains why metals are able to be pounded, extruded, bent, and shaped without breaking?
Which of the following explains why nonmetals are typically harder and more brittle than metals?
Answers
Answer: Malleability
Explanation: Is because metals have mobile electrons in their s orbitals.
Hope this helps..
Answer:
The answer to this question is mobile electrons in s orbitals.
The answer to the following question on edg is directional electrons in p orbitals.
Explanation:
If there are 8.59 x 10^22 molecules of NaCl in a salt shaker, what is the mass of NaCl?
Answers
If there are 8.59 × 10²² molecules of NaCl in a salt shaker, the mass of the substance is 8.37grams.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles of the substance by its molar mass as follows:
mass = no of moles × molar mass
However, the number of moles must be calculated from the number of molecules as follows:
no of moles = 8.59 × 10²² ÷ 6.02 × 10²³
no of moles = 1.43 × 10-¹moles
molar mass of NaCl = 58.5g/mol
mass of NaCl = 58.5 × 0.143 = 8.37g
Therefore, 8.37grams is the mass of the salt in the shaker.
Learn more about mass at: https://brainly.com/question/9651924
#SPJ1
What will happen when we replace oxygen with nitrogen in our life while breathing and what will happen if it was in our cells? (Theory no one has ever thought)
Answers
what is mass in grams of 175 mL of salt water with a density of 1.30 g/cm3
Answers
Answer:
Answer: 227.5 grams
Explanation:
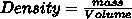
Answer:
227.5
Explanation:
Acellus
Describe how calcium and fluorine bond together to form calcium fluoride
Answers
Answer: A transfer of electrons occurs when fluorine and calcium react to form an ionic compound. This is because calcium is in group two and so forms ions with a two positive charge. ... A pairs of shared electrons makes one covalent bond. The compound formed is known as a molecule***