Answers
Related Questions
Calculate the ratio of the moles of produced to the moles of each of the reactants used. (Write two separate ratios.)
Answers
Ratio of moles of NH₃ produced to moles of N₂ used: 2 moles of NH₃ / 1 mole of N₂
Ratio of moles of NH₃ produced to moles of H₂ used: 2 moles of NH₃ / 3 moles of H₂
What is the mole ratio of the reaction?From the balanced chemical equation:
N₂ + 3 H₂ ⟶ 2 NH₃
We can determine the ratio of moles of products to the moles of each reactant.
Ratio of moles of NH₃ produced to moles of N₂ used:
From the balanced equation, we can see that 1 mole of N₂ reacts to produce 2 moles of NH₃. Therefore, the ratio is:
2 moles of NH₃ / 1 mole of N₂
Ratio of moles of NH₃ produced to moles of H₂ used:
From the balanced equation, we can see that 3 moles of H₂ react to produce 2 moles of NH₃. Therefore, the ratio is:
2 moles of NH₃ / 3 moles of H₂
Learn more about the mole ratio at https://brainly.com/question/19099163
#SPJ1
Given the equation of reaction;
N₂ + 3 H₂ ---> 2 NH₃
Calculate the ratio of the moles of produced to the moles of each of the reactants used. (Write two separate ratios.)
Is carbonic acid (H2CO3) soluble in water?
Answers
Answer:
it is soluble in water
Explanation:
mark brainliest pliiz cutee:)
Ocean acidification causes seashells to have to compete for CO3 2-, which is vital to shell formation. a. The competing reaction involves CO3 2- reacting with excess H30+ in the seawater. Write this reaction and label each conjugate acid-base pair. b. Describe how can you tell which is the acid and which is the base within the reaction
Answers
a. CO₃²⁻ + H₃O⁺ -> HCO₃⁻ + H₂O (CO₃²⁻ is the base, H₃O⁺ is the acid, HCO3- is the conjugate acid, and H₂O is the conjugate base)
b. The acid is the species that donates a proton, while the base is the species that accepts a proton.
The reaction between CO₃²⁻ and excess H₃O⁺ in seawater can be represented as:
CO₃²⁻ + H₃O⁺ -> HCO₃⁻ + H₂O
Conjugate acid-base pairs:
CO₃²⁻ / HCO₃⁻ and H₃O⁺ / H₂O
In the reaction, the species that donates a proton (H⁺) is the acid, and the species that accepts a proton is the base. In the given reaction, H₃O⁺ donates a proton to CO₃²⁻, making it the acid, while CO₃²⁻ accepts the proton to become HCO₃⁻, making it the base.
To know more about conjugate acid, here
brainly.com/question/29299518
#SPJ4
what happens when you add acetic acid vinegar and sodium bicarbonate baking soda nahco
Answers
Explanation:
oh shi sorry I wish I could help but I'm stupid
Can anyone help please.......
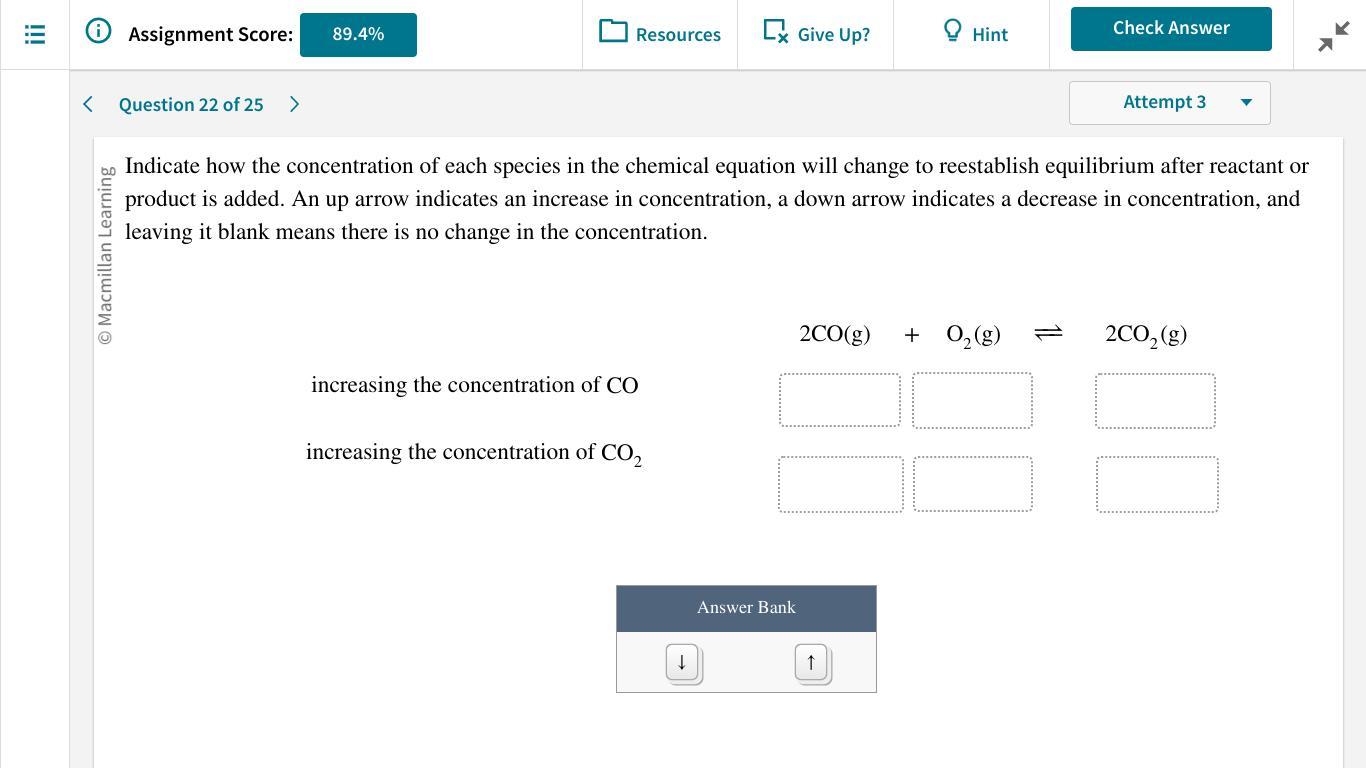
Answers
Increasing the concentration of CO decreases the equilibrium concentration of oxygen and increases the concentration of CO₂, increasing the concentration of CO₂ increases the concentration of CO and O₂.
Chemical equilibrium refers to the state of a system in which the concentration of the reactant and the concentration of the products do not change with time, and the system does not display any further change in properties.
It is the state of a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction. While a reaction is in equilibrium the concentration of the reactants and products are constant.
Learn more about Equilibrium, here:
https://brainly.com/question/30985040
#SPJ1
If I start out with 60 g of Pu-239, how much will be left after 1 half-life? How much will be left after 3 half-lives? Show your work!
Answers
There would 60 g of Pu-239 after the first half life a 30g Pu-239 and left after 3 half-lives is 7.5 g of Pu-239
Half life means the time required for half of something to undergo a process
Remind ourselves that radioactivity is defined as the process in which there is a spontaneous disintegration of a substance and in accordance of law of conservation mass that is mass is neither created nor destroyed when we have a radioactive process and as such nucleus as it is disintegrated forms daughter nuclei in which way mass is not lost
Then in this case I start out with 60 g of Pu-239 then 1 half-life is at 30g Pu-239 and after the 3 half-lives will have 7.5 g of Pu-239
Know more about Pu-239
https://brainly.com/question/29228664
#SPJ1
A silver block, initially at 55.1∘C
, is submerged into 100.0 g
of water at 25.0∘C
in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 27.9∘C
. The specific heat capacities for water and silver are Cs,water=4.18J/(g⋅∘C)
and Cs,silver=0.235J/(g⋅∘C)
.
Answers
The mass of the silver block, given that it was initially at 55.1 °C and is submerged into 100.0 g of water at 25.0°C is 189.8 g
How do i determine the mass of the silver?We'll begin our calculation by obtaining the heat absorbed by the water. Details below:
Mass of water (M) = 100 gInitial temperature (T₁) = 25 °CFinal temperature (T₂) = 27.9 °CChange in temperature (ΔT) = 27.9 - 25 = 2.9 °CSpecific heat capacity of water (C) = 4.184 J/gºC Heat absorbed by water (Q) =?Q = MCΔT
Q = 100 × 4.184 × 2.9
Q = 1213.36 J
Finally, we shall determine the mass of the silver block. Details below:
Heat absorbed by water (Q) = 6108.64 JHeat released by silver block (Q) = -1213.36 JInitial temperature of silver block (T₁) = 55.1 °CFinal temperature of silver block (T₂) = 27.9 °CChange in temperature (ΔT) = 27.9 - 55.1 = -27.2 °C Specific heat capacity of silver (C) = 0.235 J/gºC Mass of silver block (M) =?Q = MCΔT
-1213.36 = M × 0.235 × -27.2
-1213.36 = M × -6.392
Divide both sides by -6.392
M = -1213.36 / -6.392
M = 189.8 g
Thus, we can conclude that the mass of the silver block is 189.8 g
Learn more about mass:
https://brainly.com/question/1674804
#SPJ1
Complete question:
A silver block, initially at 55.1∘C, is submerged into 100.0 g of water at 25.0∘C in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 27.9∘C. The specific heat capacities for water and silver are Cs,water = 4.18J/(g⋅∘C) and Cs, silver = 0.235J/(g⋅∘C). What is the mass of the silver block?
Which graph is showing a decrease in speed?
a b c or d pls help

Answers
Answer:
D it is right ..............
Strontium hydroxide reacts with hydrobromic acid to produce Strontium bromide and
water.
Write and balance the chemical reaction above, use it for problems 1-4 below:
1. If 5.50 moles of strontium hydroxide were consumed, how much moles of water are
produced?
2. Find the mass of hydrobromic acid used to produce 7.50 moles water.
3. If 10.8 g of strontium hydroxide were used, how much moles of strontium bromide are
produced?
4. If 13.3 g of hydrobromic acid were consumed, find the mass of the water produced.
Answers
Sr(OH)2 + 2 HCl --> SrCl2 + 2 H2O
To find the moles of water produced when 5.50 moles of strontium hydroxide are consumed, we need to apply the law of conservation of mass. The mass of water produced is equal to the mass of strontium hydroxide consumed. Since strontium hydroxide has a molar mass of 142 g/mol and water has a molar mass of 18 g/mol, 1 mol of strontium hydroxide can produce 9 mol of water. Therefore, 5.50 moles of strontium hydroxide can produce 49.5 mol of water.
Similarly, 7.50 moles of water can be produced by reacting 18 moles of hydrobromic acid with strontium hydroxide. Hydrobromic acid has a molar mass of 79.9 g/mol, so 18 moles of hydrobromic acid would have a mass of 79.9 * 18 = 1435.2 g.
To find the moles of strontium bromide produced when 10.8 g of strontium hydroxide is used, we need to apply the law of conservation of mass again. The mass of the strontium bromide produced is equal to the mass of strontium hydroxide consumed. Since strontium bromide has a molar mass of 410 g/mol and strontium hydroxide has a molar mass of 142 g/mol, 1 mol of strontium bromide can consume 3.23 moles of strontium hydroxide. Therefore, 10.8 g of strontium hydroxide can produce 10.8 / 3.23 = 3.34 moles of strontium bromide.
Finally, to find the mass of water produced when 13.3 g of hydrobromic acid is consumed, we need to apply the law of conservation of mass yet again. The mass of the water produced is equal to the mass of hydrobromic acid consumed. Since hydrobromic acid has a molar mass of 79.9 g/mol, 13.3 g of hydrobromic acid would produce 13.3 / 79.9 = 0.166 moles of water.
Solve for the volume of a 1.5x10^6cm^3 object in units of liters
Answers
Answer:
1000cm³=1litre
1.5x10^6cm^3=1.5x10^6cm^3/1000litre=1.5×1000000/1000=1.5×1000=1500litres.
How many liters of carbon dioxide can be produced if 37.8 grams of carbon disulfide react with excess oxygen gas at 28.85 degrees Celsius and 1.02 atmospheres?
CS2(l) + 3O2(g) yields CO2(g) + 2SO2(g)
2.78 liters
5.95 liters
12.1 liters
11.9 liters
Answers
The volume of carbon dioxide produced is approximately (d) 11.9 liters.
To determine the amount of carbon dioxide (C\(O_2\)) produced when 37.8 grams of carbon disulfide (C\(S_2\)) reacts with excess oxygen gas (\(O_2\)), we need to use stoichiometry and the given balanced chemical equation:
C\(S_2\)(l) + 3\(O_2\)(g) → C\(O_2\)(g) + 2S\(O_2\)(g)
First, we calculate the number of moles of C\(S_2\) using its molar mass:
Molar mass of (C\(S_2\)) = 12.01 g/mol (C) + 32.07 g/mol (S) × 2 = 76.14 g/mol
Number of moles of (C\(S_2\)) = mass / molar mass = 37.8 g / 76.14 g/mol ≈ 0.496 mol
From the balanced equation, we can see that the stoichiometric ratio between (C\(S_2\)) and C\(O_2\) is 1:1. Therefore, the number of moles of C\(O_2\) produced will also be 0.496 mol.
Now we can use the ideal gas law to calculate the volume of C\(O_2\) at the given temperature and pressure. The ideal gas law equation is:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin.
Converting the temperature from Celsius to Kelvin:
T(K) = 28.85°C + 273.15 = 302 K
Using the ideal gas law:
V = nRT / P = (0.496 mol) × (0.0821 L·atm/mol·K) × (302 K) / (1.02 atm) ≈ 11.9 L
The correct answer is 11.9 liters.
for more questions on carbon dioxide
https://brainly.com/question/26150306
#SPJ8
How many moles in 3.30g of iron
Answers
The answer below is correct but to give you the process, here it is:
Molar mass of iron, Fe = 55.85 g/mol
3.30g/(55.85 g/mol) = 0.0591 mol
the grignard reagent needed to accomplish the reaction below is , and you would need 2 equivalents to synthesize the target product.
Answers
Butyryl chloride reacts with Grignard reagent, methyl magnesium bromide, to form pentan-2-one. Pentan-2-one reacts with vinyl magnesium bromide to form the final product 3-methylhex-1-en-3-ol.
Explanation-The following diagram illustrates how butyryl chloride becomes 3-methylhex-1-en-3-ol. conversion of 3-methylhex-1-en-3-ol from butyryl chloride. Tertiary alcohols are produced when carboxylic esters, R'CO2R, combine with two equivalents of an organolithium or Grignard reagent. Two identical alkyl groups are present in the tertiary alcohol (see R) A ketone intermediate is used to carry out the reaction, which is subsequently used to react with the second equivalent of the organometallic reagent. As we've just seen, the ketone undergoes further reactions to create a tertiary alcohol. This explains why, when reacting with esters, two equivalents of Grignard are required. a mechanism The Grignard performs an addition reaction on the ester in the first step, resulting in the formation of C-C and the breakdown of C-O (pi), giving us an intermediate with a negatively charged oxygen.
To know more about Grignard reagent visit:
https://brainly.com/question/19425198
#SPJ4
The pressure of a 1.7379 mol sample of Ne in a 92.202 L container is measured to be 1.4948 atm. What is the temperature of this gas in kelvins?
Answers
The temperature of a 1.7379 mole of a gas with a pressure of 1.4948 atm and volume of 92.202 L is 965.69K .
How to calculate temperature?The temperature of a given gas can be calculated using the ideal gas law equation:
PV = nRT
Where;
P = pressure = 1.4948atmV = volume = 92.202LR = gas law constant = 0.0821 Latm/molKn = number of moles = 1.7379molT = temperature1.4948 × 92.202 = 1.7379 × 0.0821 × T
137.82 = 0.142T
T = 137.82 ÷ 0.142
T = 965.69K
Therefore, the temperature of a 1.7379 mole of a gas with a pressure of 1.4948 atm and volume of 92.202 L is 965.69K.
Learn more about temperature at: https://brainly.com/question/11464844
#SPJ1
What is the name of the compound K3N
Answers
A chemical compound is a material made up of several types of molecules. Therefore, potassium Nitride is the name of the compound K\(_3\)N.
What is chemical compound?A compound is indeed a substance that is composed of two or more separate chemical elements mixed in a defined ratio in chemistry. When the elements combine, they react and generate chemical connections that really are hard to break. These bonds occur as a result of atoms sharing or exchanging electrons.
A chemical compound is a material made up of several types of molecules (or molecular entities) that include atoms from much more than one chemical element and are bound together by chemical bonds. Potassium Nitride is the name of the compound K\(_3\)N.
Therefore, potassium Nitride is the name of the compound K\(_3\)N.
To learn more about chemical compound, here:
https://brainly.com/question/26487468
#SPJ5
Which state of matter has neutral particles that bounce off one another as they collide? A: Gas B: Liquid C: Plasma D: Solid
Answers
Answer:
Gas
Explanation:
Help me please!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!

Answers
Answer:
Wave 2
Explanation:
Higher frequency means more energy per second, And wave 2 has higher frequesncy
please mark brainliest I’m almost at expert level
Answer:
I believe it is wave 2
Explanation:
As you can see in the graph, wave two happens to be more frequent. There are well, more waves than the first graph. Please forgive me if I am wrong. I tried my best to explain
how many nuetrons are in the nucleous of an atom number of 25?
Answers
Answer:
I believe there are 30
Explanation:
name the chemical compound

Answers
Answer:
hydrochloric acid
Explanation:
chemical compound any substance composed of identical molecules consisting of atoms
A 108.9 g sample of water absorbs 114.6 calories of heat. The specific heat capacity of water is 1 cal/(g·°C). By how much did the temperature of this sample change, in degrees Celsius?
Answers
Answer:
The temperature of this sample changes by 1.052 degrees Celsius
Explanation:
As we know
\(Q = mc\Delta T\)
Where m is the mass of the substance
c is the specific heat of the substance
and \(\Delta T\) is the change in temperature
Substituting the given values in above equation, we get -
\(114.6 = 108.9 * 1 * \Delta T\\\Delta T = 1.052\)degree Celsius
The temperature of this sample changes by 1.052 degrees Celsius
Calculate the amount of heat, in calories, that must be added to warm 89.7 g
of brick (0.20) from 22.0 °C
to 44.1 °C.
Assume no changes in state occur during this change in temperature.
Answers
The formula for calculating heat is:
Q = m * c * ΔT
Where Q is the heat energy required, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
Substituting the values given:
m = 89.7 g
c = 0.20 cal/g°C
ΔT = 44.1°C - 22.0°C = 22.1°C
Q = 89.7 g * 0.20 cal/g°C * 22.1°C
Q = 394.926 cal
Therefore, the amount of heat required to warm 89.7 g of brick from 22.0°C to 44.1°C is 394.926 calories.
water can be made using the reversible reaction shown, which change would kee
p this reaction from shifting to form more of the product?
Answers
We can produce more products by;
A. Increasing the concentration of H₂ gas in the reaction vessel
B. Decreasing the temperature in the reaction vessel
C. Removing the H₂O from the reaction vessel as it forms
Is formation of water an exothermic reaction?
Water is created through an exothermic process. Heat energy is released when hydrogen gas (H2) and oxygen gas (O2) mix to make water (H2O). An exothermic reaction is characterized by this energy release.
The reaction's overall energy change is negative, which shows that energy is released. The reaction is exothermic because the extra energy is released as heat into the environment.
Learn more about exothermic reaction:https://brainly.com/question/28546817
#SPJ1
Missing parts;
Water can be made using the reversible reaction shown. Which change would
keep this reaction from shifting to form more of the product?
2H₂+022H₂O + energy
A. Increasing the concentration of H₂ gas in the reaction vessel
B. Decreasing the temperature in the reaction vessel
C. Removing the H₂O from the reaction vessel as it forms
D. Increasing the temperature in the reaction vessel
The recommended application for dicyclanil for an adult sheep is 65 mg/kg of body mass. If dicyclanil is supplied in a spray with a concentration of 50. mg/mL, how many milliliters of the spray are required to treat a 70.-kg adult sheep?
Answers
Answer:
91 millilitres
Explanation:
Recommended application = 65mg / Kg
This means 65 mg of dicyclanil per kg (1 kg of body mass).
Concentration = 50 mg / mL
How many millilitres required to treat 70kg adult?
If 65mg = 1 kg
x = 70 mg
x = 70 * 65 = 4550 mg
Concentration = Mass / Volume
50 mg/mL = 4550 / volume
volume = 4550 / 50 = 91 mL
Name of [Cu[NH3)2] please fast
Answers
Name of [Cu[NH3)2] please fast
1. First name the ligand (ammine-NH3) using prefix (tetra-4) to designate number of ligands.
2. Name the central atom (copper-Cu) followed by its oxidation state (ii) in roman letter using suffix ion(since it is not neutral entity).
Explanation:
I hope it will help you...
\(kai6418\)
#CARRYONLERANING
which of the following are ways of describing solution concentrations? mole fraction percent by mass molarity molality all of the above
Answers
Mole fraction is the ratio of the number of moles of a component of a solution to the total number of moles of all components in the solution.
What is components ?Components are modular parts of a larger system or structure. They can be physical objects, such as a computer part, or abstract concepts, such as a software algorithm. Components are usually designed to work together in order to achieve a common goal. Components provide a way to break down complex tasks into smaller, manageable pieces. By combining multiple components, the overall system can be more efficient, reliable, and easier to maintain. Components are often reusable, meaning they can be used in multiple different systems or applications.
To learn more about components
https://brainly.com/question/30291912
#SPJ4
Draw the complete structure of the following tetrapeptides (draw the ionized groups in the protonated form): 1) Met- Glu-Gly-His; 2) Phe-Asp-Phe-Thr.
Answers
Answer:
See attached picture.
Explanation:
Hello,
In this case, given that each amino acid is bonded via the peptidic bond (CONH) for each tetrapeptide we obtain the structures on the attached picture. Take into account that each tetrapeptide has an initial amino side and a ending carboxylic side.
Regards.

The average speed of an oxygen molecule is 6.14 x 104 cm/sec at a certain temperature. What is the average speed of a CO2 molecule at the same temperature? (molar mass O2 = 31.9988; CO2 = 44.0098)
Answers
Answer:
the average speed of \(V_{av} CO_2\) is 5.24 × 104 cm/s
Explanation:
The computation of the average speed of a \(CO_2\) is as follows:
\(\sqrt{average} = \sqrt{\frac{8 RT}{\pi M} }\)
where,
M = Molar mass
\(V_{av} = \frac{\sqrt{1}}{M}\)
Given that
\(V_{av} \ of\ oxygen= 6.14 \times 10^4 cm/s\)
The molar mass of oxygen i.e. (MO_2) = 31.9988 g/mol
And, the molar mass of CO_2 is 44.0098 g/mol
Now
\(\frac{V_{av} CO_2}{V_{av} O_2} = \frac{\sqrt{M_{O2} }}{M_{CO2}}\)
Now place these values to the above formula
\(\frac{V_{av}CO_2}{6.14\times 104 cm/s} = \frac{\sqrt{31.9988} }{44.0098}\)
So,
V_av CO_2 is 5.24 × 104 cm/s
hence, the average speed of \(V_{av} CO_2\) is 5.24 × 104 cm/s
? I JUST DONT UNDERSTAND

Answers
Answer:
5SiO2 + 2CaC2 → 5Si + 2CaO + 4CO2
Explanation:
When you are balancing an equation, you use coefficients to change the number of atoms of each type that are on each side of the equation.
You cannot change subscripts, the small numbers to the right of the substances. You can only add coefficients, the large numbers to the left of substances.
A balanced equation has the same number and type of atoms on both sides of the equation (the reactants on the left, the products on the right).
To begin, this equation has:
1 Si
2 O
1 Ca
2 C
on the left and
1 Si
1 Ca
3 O
1 C
on the right. Those numbers don't match!
By adding coefficients we end up with 5 Si, 10 O, 2 Ca and 4 C on both sides.
Bailee explained that when traveling to the moon, he needs to pack light because the weight of objects on the moon is the same as their
weight on Earth. The mass of the objects is what changes when the gravitational pull changes. Is Bailee correct in his explanation? (1 point)
O- Bailee is incorrect. The mass of the objects stay the same but weight will change.
O- Bailee is incorrect. Both the mass and weight will change.
O- Bailee is incorrect. Both the mass and weight will stay the same.
O- Bailee is correct. The weight of the objects stay the same but mass will change.
Answers
Answer: the answer is A.
Explanation:
Bailee explained that when traveling to the moon mass of the objects is which changes when the gravitational pull changes. Bailee is incorrect. Both the mass and weight will change. Option C is correct.
What are mass and weight?Mass is the physical quantity of any substance or object and weight is the measurement of amount of force acting on a particulate body of the mass.
At the moon both the mass and weight is divided by 6 means the mass and weight is six times lighter then the expected mass and weight on earth.
Therefore, Bailee is incorrect. Both the mass and weight will change. Option C is correct. which changes when the gravitational pull changes
Learn more about mass and weight, here:
https://brainly.com/question/28122305
#SPJ1
Which of the following is not part of an amino acid?
A. ammonium group
B. carboxylate group
C. ether group
D. unique "R" group
Answers
Answer:
C
Explanation:
Ether group does not exist in amino acids
Ammonium group is on one side
the carboxylate group is opposite to the ammonium group
the R group defines what amino acid it is.