a comparison of singlet oxygen explicit dosimetry (soed) and singlet oxygen luminescence dosimetry (sold) for photofrin-mediated photodynamic therapy
Answers
SOED provides a direct measurement of singlet oxygen concentration but requires the use of specific chemical probes. SOLD, on the other hand, estimates singlet oxygen production indirectly based on the detection of its luminescence.
Singlet oxygen explicit dosimetry (SOED) and singlet oxygen luminescence dosimetry (SOLD) are two approaches used to measure the production of singlet oxygen during photofrin-mediated photodynamic therapy (PDT). Let's compare these two methods:
Singlet Oxygen Explicit Dosimetry (SOED): SOED is a technique that directly measures the concentration of singlet oxygen molecules generated during PDT. It involves the use of specific chemical probes that react selectively with singlet oxygen to produce a detectable signal. The intensity of the signal is then correlated with the amount of singlet oxygen present.
Advantages of SOED:
Direct measurement: SOED provides a direct quantification of singlet oxygen, allowing for accurate dosimetry.
Real-time monitoring: This method allows for real-time monitoring of singlet oxygen production during treatment.
Limitations of SOED:
Probe specificity: The choice of chemical probe may influence the accuracy and reliability of the measurements. Different probes may have varying reactivities or selectivities towards singlet oxygen.
Probe distribution: Ensuring uniform distribution of the probe throughout the target tissue can be challenging, potentially leading to spatial variations in singlet oxygen measurements.
Singlet Oxygen Luminescence Dosimetry (SOLD): SOLD is an indirect method that estimates the production of singlet oxygen based on the detection of its characteristic luminescence. It involves the use of photosensitizers that emit light upon interaction with singlet oxygen. The emitted light is collected and measured to estimate the singlet oxygen production.
Advantages of SOLD:
Non-invasive: SOLD does not require the introduction of exogenous probes or reagents into the tissue, making it a non-invasive technique.
Tissue penetration: The emitted luminescence can be detected even from deep within the tissue, allowing for measurements in deeper regions.
Limitations of SOLD:
Signal interpretation: The interpretation of luminescence signals can be complex due to factors such as tissue scattering and absorption, making accurate quantification challenging.
Sensitivity: SOLD measurements may be influenced by various factors, including the photosensitizer concentration, tissue oxygenation levels, and local environment.
In summary, SOED provides a direct measurement of singlet oxygen concentration but requires the use of specific chemical probes. SOLD, on the other hand, estimates singlet oxygen production indirectly based on the detection of its luminescence. The choice between these methods depends on factors such as the desired accuracy, real-time monitoring capability, and invasiveness of the technique in the context of photofrin-mediated PDT.
learn more about oxygen concentration here
https://brainly.com/question/32884654
#SPJ11
Related Questions
Potential energy stored in the bonds of a molecule is also called: __________
Answers
Potential Energy stored in bond of molecules is called chemical energy.
Define Potential Energy?Potential energy in physics is the energy that an item retains as a result of its position in relation to other objects, internal tensions, electric charge, or other elements.A potential in physics can either be a scalar potential or a vector potential. It is a field that is defined in space in either instance, and it may be used to deduce a lot of significant physical features.Potential energy is the energy that an object accumulates because of its position, enabling it to perform more work.An extended rubber band, for instance, is full of potential energy. Similar to this, a ball will have more potential energy when released above the ground than when it falls.To learn more about Potential Energy refer to:
https://brainly.com/question/14427111
#SPJ4
describe the form of the equilibrium constant expression in terms of the concentrations of the reactants and products
Answers
The equilibrium constant, Kc, is the ratio of the equilibrium concentrations of products over the equilibrium concentrations of reactants each raised to the power of their stoichiometric coefficients.
For a reaction: aA + bB→ cC + dD
Equilibrium constant: Kc=\([C]^{c}[D]^{d}\)/ \([A]^{a} [B]^{b}\)
Characteristics of Kc:
1) Equilibrium can be approached from either direction.
2) Kc does not depend on the initial concentrations of reactants and products.
3) Kc does depend on temperature.
Magnitude of Kc:
1) If the Kc value is large (Kc >> 1), the equilibrium lies to the right and the reaction mixture contains mostly products.
2) If the Kc value is small (Kc <<1), the equilibrium lies to the left and the reaction mixture contains mostly reactants.
3) If the Kc value is close to 1 (0.10 < Kc < 10), the mixture contains appreciable amounts of both reactants and products.
To know more about stoichiometric coefficients here
https://brainly.com/question/19202467
#SPJ4
hint; pay attention to the 66.2 L that's your evidence.
Question: At the maximum volume of the airbag in the simulation, how many moles of gas are contained? Use all the collected and analyzed data to explain how you determined this value.
answer using CER
Claim is your answer, evidence is from the data table, reasoning is your explanation for how you found the maximum amount of moles contained.

Answers
The answer to this question can be determined using the Ideal Gas Law, which states that PV = nRT. the maximum volume of the airbag in the simulation contains 16.6 moles of gas.
What is Ideal Gas Law?The Ideal Gas Law, also known as the Combined Gas Law, is an equation of state that describes the behavior of an ideal gas. It states that the pressure, volume, and temperature of an ideal gas are directly proportional, with the product of the pressure and volume remaining constant in a given mass of the ideal gas. In other words, when any one of these three properties is changed, the other two properties will change in an inverse manner, keeping the product constant. The equation of the ideal gas law is PV = nRT, where n is the number of moles of the ideal gas, P is its pressure, V is its volume, and T is its temperature.
Using the formula
PV= nRT
where P is pressure,
V is volume,
n is the number of moles,
R is the ideal gas constant, and
T is temperature.
Given the maximum volume of the airbag (66.2 L) and the temperature of the environment (20°C),
we can calculate the number of moles of gas contained in the airbag.
n = (P × 66.2 L) / (R ×20°C)
Using the atmospheric pressure at sea level (101,325 Pa), the calculation can be further simplified to:
n = (101,325 Pa ×66.2 L) / (8.314 J/(Kmol) × 293.15 K)
n = 16.6 mol
Therefore, the maximum volume of the airbag in the simulation contains 16.6 moles of gas.
To know more about moles, visit:
https://brainly.com/question/26416088
#SPJ1
when solid surfaces slide over each other, the kind of friction that occurs is _ friction
Answers
When solid surfaces slide over each other, the kind of friction that occurs is called Sliding Friction.
Helpppp what do i do the assisnment is
Data
Record the distance traveled for each of the trials for both floor surfaces in the table below.
Floor Surface
Data Analysis
Trial 1
Trial 2
Trial 3

Answers
Answer:
Everyday Examples of Convection
boiling water - When water boils, the heat passes from the burner into the pot, heating the water at the bottom. This hot water rises and cooler water moves down to replace it, causing a circular motion.
Explanation:
A chemical change has occured if the number of atoms is greater after the reaction than it was before the reaction.
Answers
Answer:
False
Explanation:
It is not correct to assert that a chemical change has occurred if the number of atoms is greater after the reaction than it was before the reaction.
For every chemical reaction, the law of conservation of mass must be strictly adhered.
The law states that "during a chemical reaction, matter is neither created nor destroyed, atoms are simply rearranged".
By the virtue of this law, the number of atoms at the beginning and the end of the reaction must remain the same for this law to be applicable.
Ayana rode her motorcycle down a straight freeway for 0.6hours at a constant velocity. She rode 60kilometers in that time. What was her velocity?
Answers
Answer:
velocity = 100 km/h
Explanation:
To calculate the velocity given the distance travelled and the time taken, we can use the following formula:
\(\boxed{\mathrm{velocity = \frac{distance \ travelled}{time \ taken}}}\).
In the question, we were told that Ayana travelled 60 km in 0.6 h. Using this information and the formula above, we can calculate Ayana's velocity:
velocity = \(\frac{60 \ km}{0.6 \ h}\)
= 100 km/h
Therefore, her velocity was 100 km/h.
draw the lewis structure of H2CO3
Answers
The lewis structure of the H₂CO₃ is as follows :
: O :
||
H - : O : - C - : O : - H
The H₂CO₃ is the carbonic acid. The total valence electrons in the carbonic acid that is H₂CO₃ is the 24 electrons. the carbonic acid contains the one carbon atom that is doubly bonded to the one oxygen atom and it is singly bonded to the two other oxygen atoms. The lewis structure is also called as the electron dot structure and it is as follows :
: O :
||
H - : O : - C - : O : - H
Tao learn more about lewis structure here
https://brainly.com/question/20300458
#SPJ4
A. Write the correct name for each compound below. Use prefixes to indicate the number of atoms of each element in the name of the molecular compound. (Remember: The prefix "mono-" is not used with the name of the first element.)

Answers
The compound names and their prefixes are:
Bromine trichlorideBoron nitrideDinitrogen trioxideNitrogen triiodideSulfur hexafluorideXenon tetrafluoridePhosphorus trichlorideMethanePhosphorus pentachlorideDiphosphorus pentoxideDisulfur dichlorideIodine dichlorideAmmoniaTetraphosphorus decaoxideWaterOxygen difluorideHow are molecular compounds named?Molecular compounds are named using prefixes to indicate the number of atoms of each element in the compound. The prefix for one is not used for the first element in the compound. For example, CO2 is carbon dioxide, where "di-" indicates the two oxygen atoms.
The full name of the compound is the name of the first element (using its elemental name), followed by the name of the second element (also using its elemental name) with the "-ide" suffix added, and the appropriate prefixes used to indicate the number of atoms of each element.
Learn more on molecular compounds here: https://brainly.com/question/14446156
#SPJ1
please help i will mark brainlist

Answers
Answer:
they be Cappin like a mf
Explanation:
tell the truth mf
I think the answer is 10!
A sample of oxygen was collected in a tube over water. In the tube, there is a mixture of oxygen gas and gaseous water (steam). The total pressure of the gas mixture is 760. Torr. If the pressure of gaseous water is 23. 8 torr, what is the pressure of the pure oxygen? 99. 8 torr 736 torr 740. Torr 783 torr.
Answers
Considering the Dalton's law, the pressure of the pure oxygen is 736.2 torr.
Dalton's lawThe pressure exerted by a particular gas in a mixture is known as partial pressure.
So, Dalton's law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it were alone:
\(P_{T} =P_{1} +P_{2} +...+P_{n}\)
where n is the amount of gases present in the gas mixture.
This relationship is due to the assumption that there are no attractive forces between the gases.
Pressure of the pure oxygenIn this case, you know that there is a mixture of oxygen gas and gaseous water (steam). Then the total pressure of a gas mixture is
\(P_{T} =P_{O_{2} } +P_{water}\)
You know:
the total pressure of the gas mixture is \(P_{T} =\)760 Torr. the pressure of gaseous water is \(P_{water} =\)23. 8 torr.Replacing:
\(760 torr=P_{O_{2} } +23.8 torr\)
Solving:
\(P_{O_{2} } =760 torr - 23.8 torr\)
\(P_{O_{2} } =\)736.2 torr
Finally, the pressure of the pure oxygen is 736.2 torr.
Learn more about Dalton's law:
brainly.com/question/25181467
brainly.com/question/14119417
Dear brother, please solve the q
3 - A mixture of 2kmol of CO and 3kmol of O
2
is heated to 2600 K at a pressure of 304 kPa. Given that Kp=16.461, determine the equilibrium composition of CO
2
is :
Answers
The equilibrium composition of CO₂ is determined to be 0.59 kmol.
To solve this problem, we can use the ideal gas law and the equilibrium constant expression for the reaction:
CO + 1/2O₂ ⇌ CO₂
Given the initial number of moles of CO and O₂, we can set up an ICE (Initial, Change, Equilibrium) table. Let's assume that x kmol of CO is consumed and converted to CO₂. Then, the change in the number of moles for each species is:
CO: -x kmol
O₂: -0.5x kmol
CO₂: +x kmol
At equilibrium, the number of moles of CO is (2 - x) kmol, O₂ is (3 - 0.5x) kmol, and CO₂ is x kmol. The equilibrium constant expression can be written as:
Kp = (P_CO₂) / (P_CO * P_O₂(1/2))
Given Kp = 16.461 and the pressure conditions, we can substitute the equilibrium partial pressures into the expression:
16.461 = x / ((2 - x) * (3 - 0.5x)(1/2))
Solving this equation yields x ≈ 0.59 kmol.
Learn more about equilibrium here
https://brainly.com/question/30807709
#SPJ11
4NH3+5O2-4NO+6H2O
how many moles of NH3 must react to produce 5.0 moles of NO?
Answers
Answer: 5.0 moles
Explanation:
From the equation, we see that for every 4 moles of ammonia consumed, 4 moles of nitrogen monoxide are produced (we can reduce this to moles of ammonia consumed = moles of nitrogen monoxide produced).
This means that the answer is 5.0 mol
Conduct research to examine the following factors regarding the storage of nuclear waste.
the costs, risks, and benefits to building a nuclear waste storage facility beneath Yucca Mountain
the costs, risks, and benefits to building a nuclear waste storage facility somewhere else
the costs, risks, and benefits of not building a nuclear waste storage facility at all
Based on the data you have compiled, propose an appropriate solution to this problem. Use your data to support your position on the issue.
Answers
In order to reduce the risk of radiation exposure to individuals and environmental contamination, radioactive wastes are kept. The wastes' radioactivity decreases over time.
What are the biggest problems with keeping radioactive waste in storage for a long time?Large steel and concrete barrels that contain the garbage are typically properly sealed, although accidents and leaks can still happen. Cancerous growths can result from the severe negative impacts of nuclear waste on life.
How is radioactive waste stored?Currently, dry casks are used to store all of the nuclear waste that a power plant produces over the course of its lifetime. Since 1987, Yucca Mountain in Nevada has been intended as a permanent disposal location for spent nuclear material.
To know more about radioactive wastes visit :-
https://brainly.com/question/9816140
#SPJ1
calculate the number of moles of solute in 83.85 ml of 0.1065 m k2cr2o7(aq).
Answers
0.008947 moles of solute.
To calculate the number of moles of solute, we use the formula:
moles = concentration (in mol/L) x volume (in L)
First, we need to convert the given volume of 83.85 ml to liters by dividing it by 1000:
83.85 ml ÷ 1000 ml/L = 0.08385 L
Next, we plug in the given concentration and volume into the formula:
moles = 0.1065 mol/L x 0.08385 L = 0.008947 moles
Therefore, the number of moles of solute in 83.85 ml of 0.1065 M K2Cr2O7 (aq) is 0.008947 moles.
Learn more about moles here :
https://brainly.com/question/31597231
#SPJ11
Gallium is a metal which forms compounds with a wide variety of uses. Some of the applications of gallium compounds include computer memory chips, light emitting diodes and lasers. Radioactive isotopes of gallium are used to image the human body and locate tumors. Naturally occurring gallium consists of two isotopes. One of those isotopes is 71ga with an isotopic mass of 70. 9247050 amu and an abundance of 39. 892%. What is the mass number of the other isotope?.
Answers
This problem is providing us with the isotopic mass of Ga-71 and its percent abundance, so the isotopic mass of the second isotope is required. At the end, the result turns out to be 68.92547 amu.
What is an isotope?In chemistry, isotopes are known as atoms of an element with different atomic masses (isotopic mass) but equal number of protons. In addition, they have a natural occurring abundance as a percentage.
Thus, this problem can be solved by writing the following weighted average for the atomic mass of Gallium:
\(m_{Ga}=m_{Ga-71}*\%ab_{Ga-71}+m_{Ga-?}*\%ab_{Ga-?}\)
Hence, since both percent abundances must sum 100 %, that of the second isotope will be 60.108 %. However, since its mass is unknown, one can use the average atomic mass of Gallium consigned in the periodic table in order to write the following, after plugging in what we have so far:
\(69.723=70. 9247050 amu *0.39892+m_{Ga-?}*0.60108\)
Hence, one can solve for the unknown as follows:
\(m_{Ga-?}=\frac{69.723amu-70. 9247050 amu *0.39892}{0.60108} \\\\m_{Ga-?}=68.92547amu\)
Learn more about isotopes: https://brainly.com/question/13214440
What is the term for the number of protons in the nucleus of each atom of an element?
Answers
Answer:
atomic number
Explanation:
atomic number is the number of protons
vanadium has a body-centered cubic unit cell. how many atoms of v are present in each unit cell?
Answers
The number of atoms of v are preset in each unit cell is 2.
This unit cell uses nine atoms, eight of which are corner atoms forming the cell and one further in the center of the cell. The corners contribute only one net atom and the center atom contributes another for a aggregate of two atoms.
In a BCC ( body centered cubic) unit cell, there's one atom fully contained within the unit cell at the chassis point in the center, and the chassis spots at each of the eight corners are also enthralled, but each of those is participated between eight neighboring unit cells.
So the total number of atoms for each unit cell is
= > 18/8
= > 2
To learn more about BCC:
https://brainly.com/question/14953452
#SPJ4
Question 1
Put these elements in order of the INCREASING the atomic radius.
Li, O, C, F
A)O, C, F, Li
B) Li, C, OF
C) F, O, C, Li
D) C, F, Li, o
Answers
Explanation:
we know that,
From Left to right (Period) atomic radius decreases
and
From Top to bottom (Group) atomic radius increases.
Li, C, O, F belongs to same period.
So,
Atomic radius decreases if we go left to right
Increasing order of Atomic radius -
Li > C > O > F
Therefore,
Option C is correct ✔.
Answer: C
Explanation:
Which of the following atoms cannot exceed the
octet rule in a molecule?
A) N
B) S
C) P
D) I
E) All of the atoms (a-d) can exceed the octet
rule
Answers
Of the following atoms cannot exceed the octet rule in a molecule. The answer is E) All of the atoms (A-D) can exceed the octet rule.
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons. However, there are certain atoms that can exceed the octet rule and accommodate more than eight electrons in their valence shell due to the availability of vacant d-orbitals. Atoms in the third period or beyond, such as nitrogen (N), sulfur (S), phosphorus (P), and iodine (I), can exceed the octet rule because they have d-orbitals in higher energy levels that can participate in bonding. These atoms can form expanded octets by accepting additional electrons into their d-orbitals, allowing them to accommodate more than eight electrons around the central atom in a molecule. So, all of the atoms A-D (N, S, P, and I) have the potential to exceed the octet rule and form molecules with more than eight electrons around the central atom, making option E the correct answer.
Learn more about octet rule here:
https://brainly.com/question/30779148
#SPJ11
Question 1 of 10
Match the term in Column 1 to the corresponding statement in Column 2.
BRUH CAN SOMEONE HELP!?!?!?
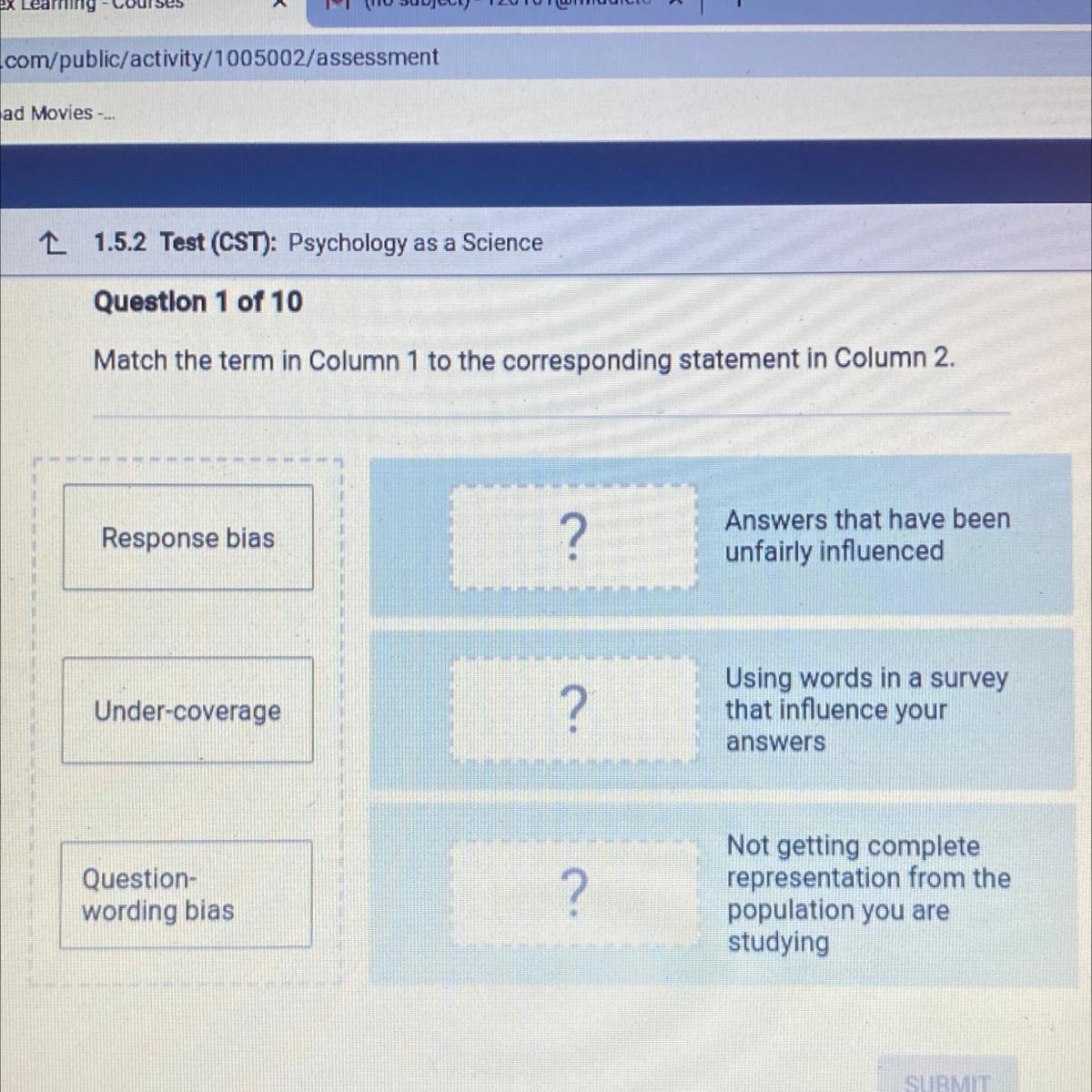
Answers
The Rescue: Firetrucks Then and Now" by Steve Otfinoski, Benchmark Books, New York (1997), a children's book reports that gun hoses can spray up to 1000 gal/min. Assume the diameter of the pipe is 2 inches to find how high the water will go straight up into the air (in meters). Determine the horsepower needed to spray the water.
Answers
The "Rescue: Firetrucks Then and Now" by Steve Otfinoski, Benchmark Books, New York (1997) reports that gun hoses can spray up to 1000 gal/min. Using the equation for flow rate (Q=A×V), the area (A) of a 2-inch pipe can be calculated to be 0.0314 m2.
To calculate the velocity (V) of the water, the equation V=Q/A can be used. This velocity can then be used to calculate the height the water will go into the air using the equation h=V²/2g, where h is the height, V is the velocity, and g is the gravitational constant.
This equation shows that the water will go 8.74 meters into the air. To calculate the power needed to spray the water, the equation P=Q×H/t can be used, where P is power, Q is flow rate, H is the height of the water in meters, and t is time in seconds.
This equation shows that the power needed to spray the water is 7.939 kW. In conclusion, the book reports that gun hoses can spray up to 1000 gal/min and using simple equations, it was determined that the water will go 8.74 meters into the air and the power needed to spray the water is 7.939 kW.
Know more about flow rate here
https://brainly.com/question/27880305#
#SPJ11
What mass of silver nitrate will react with 5.85 grams of
sodium chloride to produce 14.35 grams of silver chloride
and 8.5 grams of sodium nitrate?
Answers
I hope it help!
identify the best reagents to convert 1-hexyne into (e)-1,2-dibromo-1-hexene.select answer from the options belowxs br2, ccl41 equiv hbr, roorxs hbr1 equiv. br2, ccl41 equiv hbr
Answers
The best reagents to convert 1-hexyne into (e)-1,2-dibromo-1-hexene are 1 equiv. Br2 in CCl4, followed by NaOH to convert the mixture of (Z)- and (E)-isomers to the desired (E)-isomer.
This reaction is called the Vicinal Dibromination reaction. Option A: xs Br2 in CCl4 is a good choice of reagents, but it will give a mixture of (Z)- and (E)-isomers. Option B: 1 equiv. HBr will result in the formation of (Z)-1-bromo-1-hexene. Option C: ROOR is a radical initiator and will not result in the desired product.
To know more about different reagents and isomers : https://brainly.com/question/29713522
#SPJ11
please answer of the questions

Answers
Succession is the process of incremental alterations in the make-up of an ecological community over time. Living things occupy exposed and newly formed rock initially in primary succession.
Which is a succession example?For instance, following a forest fire that destroys all the adult trees in a certain terrain, grasses may develop, then shrubs and other tree species, until ultimately the pre-fire community is once more present. Following an interruption, such as a fire, secondary succession starts.
What does the succession process entail exactly?The term "successful" is used to refer to the process of obtaining a licence to practise law in a particular jurisdiction. There are mosses or lichens in the area where earliest species to exist. Paraphrase: They prepare that soil such that larger species, and ultimately trees, may grow there. Paraphrase: they prepare the soil.
To know more about initially visit:
https://brainly.com/question/3256540
#SPJ1
Did NASA invented thunderstorms to cover up the sound of space battles
Answers
Answer:
Nope.
Explanation:
No, I do not believe that NASA invented thunderstorms to cover up the sound of space battles. For example, Benjamin Franklin is noted to have experimented with lightning in the 1700s, well before NASA or NACA existed. Therefore, NASA did not invent the concept of thunderstorms.
if you expect the unexpected is it still unexpected??
4. Manik saw his father watering his garden plants in hot weather. He noticed that
water doesn’t stick to the plant leaves and leaves become dry but looked fresh. He asked
following questions to his teacher
a. Which tissue forms the outer covering of a plant and does it have a protective role
to play?How ?
b. Why does water not stick to the leaves?
Answers
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
What tissues protects the leaves?We know that the leaves are the parts of the plant that are involved in photosynthesis. Photosynthesis is the process by which green plants produce their own food in the presence of sunlight and chlorophyll. We know that the leave has an outer protective covering.
The tissue that plays this outer covering of a plant for is the epidermis and its waxy cuticle. It prevents damage to the plant.
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
Learn more about leaves:https://brainly.com/question/12539285
#SPJ1
Which of the following CANNOT be determined by looking at the spectra of a star? *
A:temperature
B:composition (the elements that make up the star)
C:movement toward or away from Earth
D:distance from Earth
Answers
Answer:
A:temperature
Explanation:
The temperature cannot be determined by looking at the spectra of the star due to lack of the equipment for its measurement. On the other-hand, the remaining statements like the distance from earth, movement towards or away from earth can be determined.
Elements with a high ionization energy lose electrons easily (True or False).
Answers
Answer:
False
Explanation:
What is the process called when the moon bagins to fade from full moon to new moon