Answers
Density of given block is 0.0308 g/cm³
The measure of how densely a material is packed together is called density. As the mass per unit volume, it is defined as mass per unit Volume. Unit of density is g/cm³the cubic measurement of the area taken up by a three-dimensional object is volume.The quantity of matter that makes up a thing is referred to as its mass.unit of mass gram, kilograms etc.Given,
mass of the object is 4.465g
volume of the object is 144.2cm³
we have to find out density of object in g/cm³
we know that,
Density = mass / volume
= 4.465 / 144.2 cm³
= 0.0308 g/cm³
Therefore density of object with mass of 4.465 g and volume of 144.3cm³ is 0.0308 g/cm³.
Learn more about Density here:
https://brainly.com/question/1354972
#SPJ9
Related Questions
which substance would shatter when hit with a hammer? steel, bronze, table salt, copper
Answers
Answer:
Table salt.
Explanation:
Table salt would shatter when hit with a hammer.
What is the balanced chemical
reaction for the synthesis of
carbon with hydrogen?
Answers
The balanced chemical reaction for the synthesis of carbon with hydrogen is CH₄ = C + 2H₂
Balanced chemical equation explainedBalance chemical equation refers to chemical reactions in which the moles and atoms on both the reactant side and product side are the same.
It is a way of balancing chemical equation in which the atoms of each chemicals on the product and reactant are the same.
The balanced chemical reaction for the synthesis of carbon with hydrogen is CH₄ = C + 2H₂
In this reaction, one molecule of methane is formed by combining one atom of carbon with two molecules of hydrogen gas.
Learn more about balanced chemical equation below.
https://brainly.com/question/11904811
#SPJ1
Draw the Lewis structure with the lowest formal charges for ClF2+ . Include nonzero formal charges and lone pair electrons in the structure.
Answers
Answer:
See explanation
Explanation:
The formal charge is obtained from;
Formal Charge = Valence electrons on atom - [number of bonds - lone pair electrons]
The correct structure of ClF2+ is the structure attached to this answer (image obtained from quora) in which the formal charge on fluorine is zero and the formal charge on chlorine is + 1. This is the correct structure because the chlorine is more electronegative than fluorine as expected.

The Lewis structure is a representation that shows electrons' distribution around each of the atoms composing a molecule. In the attached files you will find the Lewis structure for ClF2+.
----------------------
The Lewis structure is a representation that shows electron pairs in bonds between the atoms that compose the molecule. It also shows the solitary electron pairs that remain for each atom.
Structure
There is always a central atom and terminal atoms.
The central atom is surrounded by the terminal ones.
The central atom forms many bonds with terminal atoms.
When the molecule is composed of many atoms of the same element and a single atom of a different element, this last one is the central atom, and the others are terminal ones.
Usually, hydrogen and oxygen atoms are terminal, and carbon atoms are central.
Usually, the electropositive atoms are central, while the electronegative atoms are terminal.
Bonds
Bonds between atoms might be represented either as points or using a line. Two points equal a single line.
A pair of electrons represent one single bond.
Electrons pairs that are not involved in the bond are placed around the belonging atom.
Electrons
When adding the valence electrons of each atom we get the total number of electrons in the molecule.
The valence value is the number of electrons placed in the last energetic level of the atom.
When drawing the Lewis structure of an ion, the entire structure is between square brackets, and the charge is written as an exponent in the upper right corner, outside the brackets.
So let us analyze the exposed example, ClF2+
1) Cl and F have 6 electron pairs and a single one. 7 electrons in total each.
2) Since there are two F and one Cl, then Cl is the central atom and F are the terminal ones.
3) Valence electrons: 7e⁻ F + 7e⁻ F + 7e⁻ Cl = 21 electrons
Since the ion has a possitive charge, there are 20 electrons.
4) Electron pairs:
Each F needs to form bonds with Cl. Each bond requires two electrons.
Since there are two bonds, four electrons are required.
The 16 remaining electrons must be placed as 8 solitary pairs. Each atom needs a maximum of 8 electrons.
Each F will get 3 pairs maximum to get the 8 e⁻.Cl will get 2 pairs to get the 8 e⁻.
5) The ion is between brackets, and the charge is written as an exponent in the upper right corner.
You will find the image in the attached files.
-----------------------
You can learn more about the Lewis structure
https://brainly.com/question/20300458?referrer=searchResults
https://brainly.com/question/4144781?referrer=searchResults
https://brainly.com/question/1407731?referrer=searchResults

A student collected a sample of a gas in a 165 mL gas bulb until it’s pressure was 765 mm Hg at a temperature of 27.0 Degrees C. If the sample had a mass of 0.452 g, what is the molar mass of the gas?
Answers
The molar mass of the given gas is equal to 68.48 g.
What is the ideal gas equation?The ideal gas law can be described as an equation of the state of a hypothetical perfect gas. This equation can be represented as the product of the volume and pressure of one-mole perfect gas is equal to the product of the universal gas constant and absolute temperature of that gas.
The mathematically, ideal gas equation can be written as follows:
PV = nRT
Where n is the moles of gas, P is the pressure, V is the volume of the gas, and R is the gas constant.
Given, the volume of collected gas, V = 165 ml = 0.165 L
The temperature of the gas, T = 27° C = 273 + 27 = 300 K,
The pressure of gas, P = 765 mmHg = 0.99 atm
The value of the gas constant, R = 0.082 atm L /K mol
Substituting the values V, R, P, and T in the equation, we get:
The number of moles of the gas, n = PV/RT
n = 0.99 ×0.165/(0.082 × 300)
n = 0.0066 mol
The molar mass of the gas = 0.452/ 0.0066 =68.48 g/mol
Learn more about the ideal gas equation, here:
brainly.com/question/3637553
#SPJ1
AP chem. Sublevels!!!!!!!!!!!

Answers
Answer:
2d
Explanation:
The lowest d is 3d.
Hope this helps!
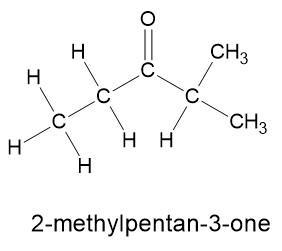
СН3-СН2-С — Н
Spell out full name
Answers
Answer:
2-methylpentan-3-one
Hope this helps.
Using this balanced equation:
NaHCO3 + CH3COOH H2O + CO2 + NaC2H3O2
In an experiment , the following mass measured 3.0 grams, 5.5 grams and 7.0 grams of
sodium bicarbonate is mixed with 0.5 mol of acetic acid (vinegar) to form carbon dioxide as
a product formed
Answers
The stoichiometric ratio of NaHCO₃ to CH₃COOH, which is determined by the balancing equation, is 1:1, meaning that 1 mole of NaHCO₃ reacts with 1 mole of CH₃COOH.
What is the balanced equation between vinegar's acetic acid CH₃COOH and sodium bicarbonate NaHCO₃?Acetic acid (CH₃COOH), a component of vinegar, interacts with sodium bicarbonate (NaHCO₃) in baking soda to produce water, carbon dioxide gas, and sodium acetate.
When NaHCO₃ and HC₂H₃O₂ are joined in a closed system, what do you suppose would happen?CO₂(g) + H₂O(l) + NaC₂H₃O₂ = NaHCO₃(aq) + HC₂H₃O₂(aq) when yellow bubbles appear, an acidic gas H₂CO₃ has generated as a result of the reaction between CO₂ and water. The salt in the solution (NaC₂H₃O₂) is basic since the solution is red in colour.
To know more about stoichiometric visit:-
https://brainly.com/question/29856106
#SPJ1
An atom is composed of a very dense central ______ containing _______, which are positively charged, and neutrons, which have _______ electric charge.
Answers
Protons, which are positively charged, and neutrons, which have no electric charge, make up the very dense core nucleus of an atom.
"An atomic nucleus contains protons, which have positive charges," In reality, the protons and neutrons that make up an atomic nucleus are called nucleons. The charge of an atomic nucleus is mostly determined by the positive charge of the protons because neutrons are neutral or have no charge.
The smallest, indivisible unit that makes up the substance that gives rise to all chemical elements is referred to as an atom. As a result, atoms are frequently thought of as the basic units of matter, the structure of which defines the nature of a chemical element.
For such more question on Protons.
https://brainly.com/question/1805828
#SPJ4
Which of the following aqueous solutions would have the greatest freezing-point depression?
(A) 0.10 m (NH₄)₂SO₄
(B) 0.10 m MnSO₄
(C) 0.10 m NaF
(D) 0.10 m KCI
(E) 0.10 m CH₃OH
Answers
A) 0.10 m (NH₄)₂SO₄ has the greatest freezing-point depression
Further explanationGiven
The aqueous solutions
Required
The greatest freezing-point depression
Solution
Colligative freezing point
\(\rm \Delta T_f=K_f\times m\)
for electrolyte :
ΔTf = Kf x m x i
i (van't Hoff factor ) = 1 + (n-1) α
All solutions in the problem have a molal concentration (0.1 m) and the same solvent ⇒ assuming water (The same Kf) so that what affects the value of ΔTf is the value of i (For methanol which is not an electrolyte, we put it aside )
Assuming the degree of electrolyte ionization α= 1, the magnitude i is determined by the number of ions produced by the electrolyte (n)
(NH₄)₂SO₄ ⇒ 2NH₄⁺ + SO₄²⁻ : 3 ions
MnSO₄ ⇒ Mn²⁺ + SO₄²⁻ : 2 ions
NaF ⇒ Na⁺ + F⁻ : 2 ions
KCl ⇒ K⁺ + Cl⁻ : 2 ions
the average human lives 74 years. how many seconds is this? write your answer in scientific notation
Answers
Number of second in human lives in scientific notation is 3.9 × 10⁷ second
Given that;
Average human lives = 74 years
Find:
Number of second in human lives in scientific notation
Computation:
Number of second in human lives in scientific notation = 74 × 365 × 24 × 60
Number of second in human lives in scientific notation = 38,894,400
Number of second in human lives in scientific notation = 3.9 × 10⁷ second
Learn more:
https://brainly.com/question/3712546?referrer=searchResults
9. Given the following reaction:CO (g) + 2 H2(g) CH3OH (g)In an experiment, 0.45 mol of CO and 0.57 mol of H2 were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.28 mol of CO remaining. Keq at the temperature of the experiment is ________.
Answers
Answer:
\(Keq=11.5\)
Explanation:
Hello,
In this case, for the given reaction at equilibrium:
\(CO (g) + 2 H_2(g) \rightleftharpoons CH_3OH (g)\)
We can write the law of mass action as:
\(Keq=\frac{[CH_3OH]}{[CO][H_2]^2}\)
That in terms of the change \(x\) due to the reaction extent we can write:
\(Keq=\frac{x}{([CO]_0-x)([H_2]_0-2x)^2}\)
Nevertheless, for the carbon monoxide, we can directly compute \(x\) as shown below:
\([CO]_0=\frac{0.45mol}{1.00L}=0.45M\\\)
\([H_2]_0=\frac{0.57mol}{1.00L}=0.57M\\\)
\([CO]_{eq}=\frac{0.28mol}{1.00L}=0.28M\\\)
\(x=[CO]_0-[CO]_{eq}=0.45M-0.28M=0.17M\)
Finally, we can compute the equilibrium constant:
\(Keq=\frac{0.17M}{(0.45M-0.17M)(0.57M-2*0.17M)^2}\\\\Keq=11.5\)
Best regards.
hich of the following is not true of Christian art after the Edict of Milan?
a.
The art included Christian symbols, such as the cross and the Good Shepard.
b.
Mosaics freely covered church walls and ceilings.
c.
Sculptures rarer than paintings.
d.
Artists used bright colors and shading.
Answers
Answer:
Sculptures rarer than paintings.
Explanation:
Answer:
C. Sculptures rather than paintings
Explanation:
Briefly explain the concept of heat transfer with examples
Answers
Answer:
Heat transfer by radiation occurs when microwaves, infrared radiation, visible light, or another form of electromagnetic radiation is emitted or absorbed. An obvious example is the warming of the Earth by the Sun. A less obvious example is thermal radiation from the human body.
oxygen and sulfur bonding patterns: build each of the indicated molecules below in the simulator and match them to the correct bonding pattern in respect to either oxygen or sulfur. remember if a molecule does not have a name when you build it is not a stable arrangement and you might have to reorder your atoms. for example: h2o - 2 single bonds as you complete the matching below think of the following: how do oxygen and sulfur atoms tend to bond in molecules? what patterns do you see? how many covalent bonds (total) do oxygen atoms or sulfur atoms tend to form? group of answer choices ocl2 [ choose ] no [ choose ] onh [ choose ] co2 (only describe one of the oxygen atoms) [ choose ] bs [ choose ] sis2 (only describe one of the sulfur atoms) [ choose ]
Answers
The compromise for these two elements would be to share valence electrons in order for them to adhere to the octet rule. Nonmetallic elements oxygen and Sulphur fall into this category. Covalent bonds are produced when atoms share electrons.
What is meant by element?Chemical substances that cannot be converted into other chemicals are referred to as elements.Chemical elements are distinguishable from one another by the number of protons in their atoms' nucleus, which is the fundamental particle that makes up a chemical element. A crucial component of a whole. a simple substance that cannot be divided into smaller components or transformed into another substance is referred to as in chemistry. Atoms, which are made up of protons, neutrons, and electrons, are the building blocks of an element. One element has a fixed number of protons in each of its atoms. A basic thing that is difficult to divide into smaller parts is known as an element.To learn more about element, refer to:
https://brainly.com/question/18096867
#SPJ4
the libretto of doctor atomic includes excerpts from the hindu bhagavad gita, even though oppenheimer was unfamiliar with that text. T/F
Answers
The libretto of doctor atomic includes excerpts from the Hindu Bhagavad gita, even though Oppenheimer was unfamiliar with that text. The statement is false.
What is the libretto of doctor atomic?The libretto of Doctor Atomic was created by Peter Sellars, drawing on original source material, including personal memoirs, recorded interviews, technical manuals of nuclear physics, declassified government documents, and the poetry of Muriel Rukeyser, an American poet and contemporary of Oppenheimer.
The storyline of Doctor atomic has it that scientists and soldiers were secretly stationed in Los Alamos, New Mexico, for the duration of World War II as they worked to bring a monstrous weapon to life.
Hence, Doctor Atomic focuses on the days and hours leading up to the first test of the bomb on July 16, 1945.
Learn more about Oppenheimer at: https://brainly.com/question/519587
#SPJ1
you are presented with a solution. when solute crystals are added to the solution, they sink and remain on the bottom of the beaker. the solution is said to be group of answer choices undetermined saturation supersaturated unsaturated saturated
Answers
The given solution is a saturated solution. A homogeneous mixture composed of two or more substances is called a solution.
In a solution, there are two primary components, which are termed as,
Solute (The minor component that is dissolved in a solution).Solvent (The major component that dissolves the solute).At a given temperature and pressure, every solvent is capable of dissolving a particular amount of solute in it.
If a solution has dissolved less amount of solute than it is capable of dissolving, then the solution is known as an unsaturated solution.
If a solution has dissolved as much as it is capable of dissolving, then the solution is known as a saturated solution.
If a solution has dissolved more amount of solute than it is capable of dissolving, then the solution is known as a supersaturated solution.
In the given problem, when the solute crystals are added to the solution, they sink and remain on the bottom of the beaker.
Hence, the given solution is saturated.
Learn more about the saturated solutions here: brainly.com/question/2078413
#SPJ4
Consider the following reaction in chemical equilibrium: 2BrNO(g)⇌2NO(g)+Br2(g) What is the effect of adding additional Br2 (g) to the reaction mixture? What is the effect of adding additional BrNO (g)? Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. ResetHelp Adding BrNO Adding B r N O blank the concentration of B r N O , causing the reaction progress to shift to the blank (away from the blank). the concentration of BrNO , causing the reaction progress to shift to the Adding B r N O blank the concentration of B r N O , causing the reaction progress to shift to the blank (away from the blank). (away from the Adding B r N O blank the concentration of B r N O , causing the reaction progress to shift to the blank (away from the blank).). Adding Br2 Adding B r 2 blank the concentration of B r 2 , causing the reaction progress to shift to the blank. the concentration of Br2 , causing the reaction progress to shift to the Adding B r 2 blank the concentration of B r 2 , causing the reaction progress to shift to the blank..
Answers
Answer:
Adding of Br2 to the reaction mixture favours/increases the concentration of BrNO, causing the reaction progress to shift to the left/reactants (away from the forward reaction/products).
Adding of BrNO to the reaction mixture favours/increases the concentration of Br2 and NO (the products), causing the reaction progress to shift to the right/products (away from the backward reaction/reactants).
Explanation:
2BrNO(g) ⇌ 2NO(g) + Br2(g)
According to the Le Chatellier's principle, when one of the factors involved the equilibrium (concentration, pressure and temperarure) of a chemical reaction changes, the equilibrium position of the reaction shifts to remedy this change.
When the concentration of one of the reactants or products changes, the whole position of equilibrium of the reaction shifts to account for this change.
From the reaction given in the question, 2BrNO is on the reactant side and 2NO & Br2 are on the product side.
If more of Br2 is then added to the reaction, it causes an overload of Br2, a product of the reaction, in the reaction setup and the equilibrium responds by shifting to the left, favouring the backward reaction and leading to the production of more BrNO (reactants).
If more of BrNO is then added to the reaction, it causes an overload of BrNO (a reactant in the reaction) in the reaction setup and the equilibrium responds by shifting to the right, favouring the forward reaction and leading to the production of more Br2 and NO (products).
Hope this Helps!!!
The scientific method is the way scientists solve problems. Scientists begin to solve a problem by making _____.
Answers
Answer:
Explanation: observations
PLEASEEE HELPPP MEEEEE WITH THIS QUESTIONNN!!!!!
I REALLY NEED A GREAT RESPONSE AND ILL MARK YOU BRAINLIEST FOR YOUR EFFORT!!!!!!!
QUESTION: THINK CRITICALLY: Some conifers have female cones on the top half of the tree and male cones on the bottom half. Why would this arrangement of cones on a tree be important?
Answers
Answer:
The male cones appear on the trees in the spring time. The male cones are smaller than the female cones that we typically think of when we think of pine cones. These cones are softer and are only on the trees in the spring. After they release their pollen they die away and disappear.
Explanation:
Determine the pH at the point in the titration of 40.0 mL of 0.200 M H₂NNH₂ with 0.100 M HNO₃ after 100.0 mL of the strong acid has been added. The value of Kb for H₂NNH₂ is 3.0 × 10⁻⁶.
Answers
Answer:
pH = 1.85
Explanation:
The reaction of H₂NNH₂ with HNO₃ is::
H₂NNH₂ + HNO₃ → H₂NNH₃⁺ + NO₃⁻
Moles of H₂NNH₂ and HNO₃ are:
H₂NNH₂: 0.0400L ₓ (0.200mol / L) = 8.00x10⁻³ moles of H₂NNH₂
HNO₃: 0.1000L ₓ (0.100mol / L) = 0.01 moles of HNO₃
As moles of HNO₃ > moles of H₂NNH₂, all H₂NNH₂ will react producing H₂NNH₃⁺, but you will have an excess of HNO₃ (Strong acid).
Moles of HNO₃ in excess are:
0.01 mol - 8.00x10⁻³ moles = 2.00x10⁻³ moles of HNO₃ = moles of H⁺
Total volume is 100.0mL + 40.0mL = 140.0mL = 0.1400L.
Thus, [H⁺] is:
[H⁺] = 2.00x10⁻³ moles / 0.1400L = 0.0143M
As pH = - log [H⁺]
pH = 1.85The pH of the resulting solution is 1.85.
The reaction is 1:1 hence;
Number of moles of H₂NNH₂ = 40/1000 L × 0.200 M = 0.008 moles
Number of moles of HNO₃ = 100/1000 L × 0.100 M = 0.01 moles of HNO3
Number of moles of excess acid = 0.01 moles - 0.008 = 0.002 moles
Total volume of solution;
40.0 mL + 100.0 mL = 140 mL or 0.14 L
Molarity of excess acid = 0.002 moles/ 0.14 L = 0.014 M
Since;
pH = -log[H^+]
pH = -log[0.014 M]
pH = 1.85
The pH of the resulting solution is 1.85.
Learn more: https://brainly.com/question/20906233
The addition of 0.242 L of 1.92 M KCl to a solution containing Ag+ and Pb2+ ions is just enough to precipitate all of the ions as AgCl and PbCl2. The total mass of the resulting precipitate is 65.08 g. Find the mass of PbCl2 and AgCl in the precipitate. Calculate the mass of PbCl2 and AgCl in grams.

Answers
Answer:
Mass PbCl₂ = 50.24g
Mass AgCl = 14.84g
Explanation:
The addition of Cl⁻ ions from the KCl solution results in the precipitation of AgCl and PbCl₂ as follows:
Ag⁺ + Cl⁻ → AgCl(s)
Pb²⁺ + 2Cl⁻ → PbCl₂(s)
If we define X as mass of PbCl₂, moles of Cl⁻ from PbCl₂ are:
Xg × (1mol PbCl₂/ 278.1g) × (2moles Cl⁻ / 1 mole PbCl₂) = 0.00719X moles of Cl⁻ from PbCl₂
And mass of AgCl will be 65.08g-X. Moles of Cl⁻ from AgCl is:
(65.08g-Xg) × (1mol AgCl/ 143.32g) × (1mole Cl⁻ / 1 mole AgCl) = 0.45409 - 0.00698X moles of Cl⁻ from AgCl
Moles of Cl⁻ that were added in the KCl solution are:
0.242L × (1.92mol KCl / L) × (1mole Cl⁻ / 1 mole KCl) = 0.46464 moles of Cl⁻ added.
Moles Cl⁻(AgCl) + Moles Cl⁻(PbCl₂) = Moles Cl⁻(added)
0.45409 - 0.00698X moles + (0.00719X moles) = 0.46464 moles
0.45409 + 0.00021X = 0.46464
0.00021X = 0.01055
X = 0.01055 / 0.00021
X = 50.24g
As X = Mass PbCl₂
Mass PbCl₂ = 50.24gAnd mass of AgCl = 65.08 - 50.24
Mass AgCl = 14.84gThe masses of the compounds in the precipitate can be found my knowing
the number of moles of chloride ion contributed by each compound.
The mass of PbCl₂ in the precipitate is approximately 49.24 gThe mass of AgCl in the precipitate is approximately 15.84 gReasons:
The given parameter are;
Volume of KCl solution added = 0.242 L
Concentration of KCl solution = 1.92 M KCl
The ions in the solution to which KCl is added = Ag⁺ and Pb²⁺ ions
Precipitates formed = AgCl and PbCl₂
The mass of the precipitate = 65.08 g
Required:
The mass of PbCl₂ and AgCl in the precipitate
Solution;
Number of moles of chloride ions in a mole of PbCl₂ = 2 moles
Number of moles of chloride ions in a mole of AgCl = 1 mole
Let X represent the mass of PbCl₂ in the precipitate, we have;
The mass of AgCl in the precipitate = 65.08 g - X
\(\mathrm{Number \ of \ moles \ of \ PbCl_2} = \dfrac{X \, g}{278.1 \, g} =\mathbf{ \dfrac{X }{278.1}}\)
Number of moles of chloride ions from PbCl₂ is therefore;
\(\mathrm{Number \ of \ moles \ of \ Cl^- from \ PbCl_2} =\mathbf{ 2 \times \dfrac{X }{278.1} \ moles \ of \ Cl^-}\)
\(\mathrm{Number \ of \ moles \ of \ AgCl \ in \ the \ precipitate} = \dfrac{65.08 -X }{143.32}\)
\(\mathrm{Number \ of \ moles \ of \ Cl^- from \ AgCl} = \mathbf{ \dfrac{65.08 -X }{143.32}} \ moles \ of \ Cl^-\)
The number of moles of chloride ions from one mole of KCl = 1 mole
Number of moles of chloride ions from 0.242 L of 1.92 M KCl is therefore;
0.242 L × 1.92 moles/L = 0.46464 moles
Number of moles of chloride ions from KCl = 0.46464 moles
\(0.46464 \ moles \ from \ KCl = \overbrace{ \dfrac{ 2 \times X }{278.1} + \dfrac{65.08 -X }{143.32}} \ moles \ in \ PbCl_2 \ and \ AgCl\)
Which gives;
\(\displaystyle \frac{192}{896089} \cdot X + \frac{1627}{3583} = \frac{1452}{3125}\)
Therefore;
\(\displaystyle X = \frac{\frac{1452}{3125} - \frac{1627}{3583} }{ \frac{192}{896089} } = \frac{105864850549}{2149800000} \approx \mathbf{ 49.24}\)
The mass of PbCl₂ in the precipitate, X ≈ 49.24 g
The mass of AgCl in the precipitate = 65.08 g - 49.24 g ≈ 15.84 g
Learn more here:
https://brainly.com/question/13652772
Measurement of several of the air pollutants by the EPA includes an amount measurement as well as a ______ measurement.
D. width
C. time
B. length
A. scent
Answers
C:time
Explanation:
time
miscible liquids that have different boiling points can be separated by
Answers
Answer:
Distillation.
Explanation:
Hello there!
In this case, by considering the attached file, which depicts the process of distillation, whereby miscible liquids are separated out by firstly boiling and consequently condensing the liquid with the lowest boiling point so that the liquid with the highest boiling point remains in the flask. This process is widely used in the oil gas industry as lots of heavy (high boiling points) and light (low boiling points) produces can be obtained via distillation.
Regards!

a
46. Make and Use Tables Find information in
newspaper articles or magazines describing
five recent earthquakes. Construct a table for
each earthquake that shows the date, location,
magnitude, and whether the damage caused
by the earthquake was light, moderate, or
heavy.
Answers
Answer:
the one above me is correct
Predict the sign of ∆H for placing ice on your lab table. Is this reaction endothermic or exothermic? Explain.
Answers
Answer:
Explanation:
Placing ice on a lab table is an exothermic process, which means that the enthalpy change (∆H) for this reaction is negative.The reason for this is that when ice is placed on a lab table, it comes into contact with a surface that is warmer than its own temperature, and heat flows from the warmer surface (the lab table) to the cooler surface (the ice). This heat transfer causes the ice to melt, which is an exothermic process since heat is released to the surroundings.Since exothermic processes release heat energy, the enthalpy change (∆H) for this reaction is negative. Therefore, placing ice on a lab table has a negative ∆H, indicating that it is an exothermic process.
Given the following equation: Mg + 2HCI → MgCl₂ + H₂
How many moles of H₂ can be produced by reacting 2 moles
of HCI?
Answers
Taking into account the reaction stoichiometry, 1 mole of H₂ can be produced by reacting 2 moles of HCI.
Reaction stoichiometryIn first place, the balanced reaction is:
Mg + 2 HCl → MgCl₂ + H₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Mg: 1 moleHCl: 2 molesMgCl₂: 1 moleH₂: 1 moleMoles of H₂ producedBy reaction stoichiometry 2 moles of HCl form 1 mole of H₂.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
What is the mass of 2.7 x 10^18 molecules of HBr? (molar mass of HBr = 80.912 g/mol)
Answers
Answer:3.6 X 10-4 g
Explanation:
2.7 X 10^18 X (1 mole / (6.02 X 10^23) X ( 80.912 g / 1 mole) = 3.629 X 10-4 g HBr. If needed to correct Sig Figs 3.6 X 10-4 g HBr
PLEASE ANSWER SOON AS POSSIBLE
What is the volume, in liters, of 1.20 mol of C3H8 gas at STP
Answers
26.8L is the volume, in liters, of 1.20 mol of C\(_3\)H\(_8\) gas at STP. A measurement of three-dimensional space is volume.
A measurement of three-dimensional space is volume. It is frequently expressed quantitatively using SI-derived units, like the cubic metre or litre, or different imperial or US-standard units, including the gallon, quart and cubic inch. Volume and length (cubed) have a symbiotic relationship.
The volume of an envelope is typically thought of as its capacity, not as the amount of space it takes up. In other words, the volume is the quantity of fluid (liquid or gas) that the container may hold.
Volume = 1.20×22.4
=26.8L
To know more about volume, here:
https://brainly.com/question/1578538
#SPJ1
in the following chemical reaction between H_2 and Cl_2 to produce HCl, what is the sum of the mass of HCl produced plus the mass of left over reactants when 0.40 g of H_2 completely reacts with 12.35 g of Cl_2?
H_2(g) + Cl_2(g) → 2HCl(g)
Answers
Answer:
Left over mass of hydrogen = 0.06 g
Mass of HCl produced = 12.41 g
Explanation:
Given data:
Mass of H₂ = 0.40 g
Mass of Cl₂ = 12.35 g
Mass of left over reactant = ?
Mass of HCl produced = ?
Solution:
Chemical equation:
H₂ + Cl₂ → 2HCl
Number of moles of H₂:
Number of moles = mass / molar mass
Number of moles = 0.40 g/ 2 g/mol
Number of moles = 0.2 mol
Number of moles of Cl₂:
Number of moles = mass / molar mass
Number of moles = 12.35 g/ 71 g/mol
Number of moles = 0.17 mol
Now we will compare the moles of HCl with H₂ and Cl₂.
H₂ : HCl
1 : 2
0.2 : 2×0.2 = 0.4
Cl₂ : HCl
1 : 2
0.17 : 2 × 0.17 = 0.34
Chlorine is limiting reactant.
Mass of HCl produced:
Mass = number of moles × molar mass
Mass = 0.34 mol × 36.5 g/mol
Mass = 12.41 g
Leftover mass of hydrogen:
Cl₂ : H₂
1 : 1
0.17 : 0.17
Number of moles of H₂ react with Cl₂ are 0.17.
Moles remain unreacted = 0.2 - 0.17 = 0.03 mol
Mass left over:
Mass = number of moles × molar mass
Mass = 0.03 mol × 2 g/mol
Mass = 0.06 g
The sum of the mass of HCl produced plus the mass of left over reactants is:
Mass of hydrogen = 0.06 g
Mass of HCl = 12.41 g
Chemical ReactionGiven:
Mass of H₂ = 0.40 g
Mass of Cl₂ = 12.35 g
Mass of left over reactant = ?
Mass of HCl produced = ?
Chemical equation: H₂ + Cl₂ → 2HClNumber of moles of H₂:
Number of moles = mass / molar mass
Number of moles = 0.40 g/ 2 g/mol
Number of moles = 0.2 mol
Number of moles of Cl₂:
Number of moles = mass / molar mass
Number of moles = 12.35 g/ 71 g/mol
Number of moles = 0.17 mol
The moles of HCl with H₂ and Cl₂.
H₂ : HCl
1 : 2
0.2 : 2×0.2 = 0.4
Cl₂ : HCl
1 : 2
0.17 : 2 × 0.17 = 0.34
The chlorine is limiting reactant.
Mass of HCl produced:
Mass = number of moles × molar mass
Mass = 0.34 mol × 36.5 g/mol
Mass = 12.41 g
Leftover mass of hydrogen:
Cl₂ : H₂
1 : 1
0.17 : 0.17
Number of moles of H₂ react with Cl₂ are 0.17.
Moles remain unreacted = 0.2 - 0.17 = 0.03 mol
Mass left over:
Mass = number of moles × molar massMass = 0.03 mol × 2 g/molMass = 0.06 gLearn more about "Moles":
https://brainly.com/question/7287712?referrer=searchResults
A lab group was calculating the speed of a radio car. They measured the distance traveled to be 6 meters and the time to be 3.5 seconds. Then they divided the distance by the time to find the speed. The actual speed was 2.2 m/s. What was their percent error?
Answers
Answer:456
Explanation: