A 6.40 g sample of a compound is burned to produce 8.37 g CO_2, 2.75 g H_2O, 1.06 g N_2, and 1.23 g SO_2. What is the empirical formula of the compound? Give your answer in the form C#H#N#O#S# where the number following the element’s symbol corresponds to the subscript in the formula. (Don’t include a 1 subscript explicitly).
Answers
The empirical formula :
C₁₀H₁₆N₄SO₇
Further explanationGiven
6.4 g sample
Required
The empirical formula
Solution
mass C :
= 12/44 x 8.37 g
= 2.28
mass H :
= 2/18 x 2.75 g
= 0.305
mass N = 1.06
mass S :
= 32/64 x 1.23
= 0.615
mass O = 6.4 - (2.28+0.305+1.06+0.615) = 2.14 g
Mol ratio :
= C : H : N : S : O
= 2.28/12 : 0.305/1 : 1.06/14 : 0.615/32 : 2.14/16
= 0.19 : 0.305 : 0.076 : 0.019 : 0.133 divided by 0.019
= 10 : 16 : 4 : 1 : 7
The empirical formula :
C₁₀H₁₆N₄SO₇
Related Questions
4. Circle the element with the greatest atomic radius. [4]
C.
a.
sodium or magnesium
b. magnesium or beryllium
lithium or rubidium
d. cesium or radon
oxygen or fluorine
f. phosphorus or aluminum
g. calcium or barium
h. boron or gallium
e.
Answers
The atomic greatest radius is magnisium
Jade wanted to test the effect of ice on the weathering of rocks. She filled two containers with gypsum and placed a water balloon in one of the containers. She put both containers in a freezer for few hours and cut open the containers. She found out that the gypsum block with the water balloon cracked while the other block did not. How is this similar to the weathering of rocks by ice?
two blocks of gypsum, one has a water balloon in it and is cracked
The gypsum block cracks, just like rocks when the temperature increases.
The water balloon pops, causing the water to flow just like rain flows over rocks.
The water contracts as it freezes, just like the water seeps into the cracks of rocks.
The water in the balloon freezes and expands, just like the water in the cracks of rocks.
Answers
She found out that the gypsum block with the water balloon cracked while the other block did not.The water in the balloon freezes and expands, just like the water in the cracks of rocks - is this similar to the weathering of rocks by ice.
What is gypsum?Gypsum is a soft sulfate mineral that is made up of calcium sulfate dihydrate and has the chemical formula CaSO₄ . 2H₂O. It is extensively mined, and used as fertilizer, the primary ingredient in many types of plaster, sidewalk or blackboard chalk, and drywall, and it is also widely utilized as a building material.
In nature, water flows from small rivers and occasionally through fissures in the rocks or terrain, and when it freezes, it puts too much pressure on the rock, causing it to split. The similarity in Jades' test is that the water balloon has water when it is placed in the gypsum, and when she adds a cold environment, the ice within the water balloon freezes and expands, putting too much pressure on the rock, causing it to shatter.
Ice Wedging is a example of this.
To know more about gypsum refer to:
https://brainly.com/question/9049646
#SPJ1
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
Question 14www3: HW 8-9 (Due August 3)0/1 pt10 DetailsHW 8-Acids and BasFollow these steps to write the balanced chemical equation for the reaction between acetic acid and sodiumhydroxide.1. Start by writing the proper names of the products below (separated by a + sign).Acetic acid + Sodium hydroxide →2. Next, write the full, balanced molecular equation with the correct chemical formulas, includingcoefficients.3. Finally, write the net ionic equation for this reaction below, including ionic charges.
Answers
Answer
1. Acetic acid + Sodium hydroxide → Sodium Acetate + Water
2. CH₃COOH + NaOH → CH₃COONa + H₂O
3. CH₃COOH + OH⁻ → CH₃COO⁻ + H₂O
Explanation
1. The proper names of the products for the reaction between acetic acid and sodium hydroxide are given below (separated by a + sign).
Acetic acid + Sodium hydroxide → Sodium Acetate + Water
2. The full, balanced molecular equation with the correct chemical formulas, including coefficients is:
CH₃COOH + NaOH → CH₃COONa + H₂O
3. Finally, the net ionic equation for this reaction is below, including ionic charges.
Strong bases are considered strong electrolytes and will dissociate completely. This means that NaOH will split apart in the net ionic equation. Weak acids only dissociate partially and are not considered to split apart into ions when writing net ionic equations.
CH₃COOH + Na⁺ + OH⁻ → CH₃COO⁻ + Na⁺ + H₂O
Cross the spectator ions, we have:
CH₃COOH + OH⁻ → CH₃COO⁻ + H₂O
You add 8.5 g of iron to 27.90 mLof water and observe that the volume of iron and water together is 28.98 mL calculate the density of iron
Answers
Answer: 7.87 g/ml
Explanation:
(28.98 ml - 27.90ml) = 7.87 g/ml
What would happen if there were no gravity anywhere in the universe?
Answers
Objects would no longer be drawn toward each other, because there would be no sloping surface for them to fall down. Instead, they would fly off in whatever direction gravity was keeping them from going.
Answer:
All items in the universe, humans, objects, and the planets, would be floating around. Everything would become weightless.
A substance in a specific state of matter was transferred from a cylindrical-shaped container to a cube-shaped container. The substance took different shapes in each container. Which of following could be another characteristic of the substance?
a) partially compressible
b) Its particles are arranged in a fixed pattern
c) It has very strong intermolecular forces between particles
Answers
Partially compressible is the right answer because the substance is a liquid.
How the substance is Partially compressible?If the substance took different shapes in each container, this means that it can be partially compressible like liquids. We know that liquids have a definite volume and take the shape of the container so we can conclude that Partially compressible is the right answer.
Learn more about liquid here: https://brainly.com/question/25664350
A 32.3-gram sample of gas is found to have a volume of 1.9 liters at 301 K and 1.21 atm. What is the molar mass of this gas? Show all of the work used to solve this problem.
Answers
Answer:
351.1g/mol
Explanation:
you can find the answer using The ideal gas equation
n= PV/RT
n=(1.21*1.9/0.082*301)mol
n=0.092 mol
molar mass=Mass/mole
m=32.3g/0.092mol
m=351.1g/mol
Complete the following sentences to identify the process that ice, water, or water vapor may undergo if either the temperature or the pressure is increased.
Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.
FREEZE 1. If ice is heated at a constant pressure of 0.00512 atm, it will_____________.
CONDENSE 2. If ice is heated at a constant pressure of 1 atm, it will_____________.
MELT 3. If the pressure of water vapor is increased at a constant pressure of 100 degrees Celsius,it will____.
SUBLIME 4. If the pressure of water vapor is increased at a constant pressure of -50 degrees Celsius,it will__.
VAPORIZE
DEPOSIT
Answers
Answer:
Freeze
Condense
Melt
Sublime
Explanation:
a uniform electric field of magnitude 352 n/c pointing in the positive x-direction acts on an electron, which is initially at rest. the electron has moved 3.10 cm.
Answers
The work performed on an electron by the field is 0.18 × 10⁻¹⁸J.
An electric field that has a constant field strength value throughout is said to be uniform.
The product of the electron charge q and the electric field intensity determines the force that the electric field applies to the electron.
F: qE = ( 1.6 × 10⁻¹⁹) (375 N/ C) = 6 × 10⁻¹⁷ N
The electron moves by the following distance when this force is at work: x = 3.10 cm = 0.031 m
Additionally, the product of the force's magnitude and the electron's displacement represents the work that the electric field does on the electron. Since the force's direction matches the electron's displacement, the sign must be interpreted as positive, hence
W = FΔx = ( 6 × 10⁻¹⁷ N) (0.031 m ) = 0.18 × 10⁻¹⁸ J
To know about electron
https://brainly.com/question/1255220
#SPJ4
Using C2H4 + 3 O2 -> 2 CO2 + 2 H2O. If 20 moles of fuel are combusted in the above equation, how many moles of O2 are consumed?
Answers
Answer:
Using C2H4 + 3 O2 -> 2 CO2 + 2 H2O. If 20 moles of fuel are combusted in the above equation, how many moles of O2 are consumed?
Explanation:
According to the balanced chemical equation:
1 mole of C2H4 reacts with 3 moles of O2
Therefore, for 20 moles of C2H4 combusted, we would need:
20 moles C2H4 × (3 moles O2 / 1 mole C2H4) = 60 moles O2
So, 60 moles of O2 are consumed in the combustion of 20 moles of C2H4.
What is true about atoms and molecules?
Select the correct answer below:
Molecules are very heavy but atoms are very light.
Atoms are always gases and molecules are always solids.
Molecules are always smaller than atoms.
Atoms and molecules are both very small and light.
Answers
Molecules are very heavy but atoms are very light is true about atoms and molecules.
How do molecules and atoms vary from one another?Individually neutral particles make up an atom. The bonding of two or more atoms forms molecules, which are neutral entities. An ion is a particle that is positively or negatively charged.
The capacity of water molecules to attract other water molecules is referred to as cohesion, and this trait makes water a "sticky" liquid.
A group of two or more atoms bound together by the attractive forces known as chemical bonds is referred to as a molecule; depending on the context, the word may or may not include ions that meet this requirement.
learn more about Molecules
https://brainly.com/question/475709
#SPJ1
1. An electric iron has a
power rating of 750W
a. How many joules of
electric energy does it
change into heat energy
every second?
b. How many joules of
work can it do in 3
seconds
c. How long does it take
the iron to do 1500J of
work?
2. Use the kinetic particle
theory to explain why a
solid has a definite shape
and liquid has none.
Answers
Explanation:
a) E = P × t
E = 750 × 1 s = 750 J
b) E = P × t
E = 750 × 3s = 2250 J
c) E = P × t
1500 = 750 × t
t = 1500/750
t = 2 s
Why does it mean by methane molecule is symmetrical?
Answers
A methane molecule (CH4) is considered symmetrical because it possesses a symmetric structure and exhibits symmetry operations.
Symmetry refers to a balanced arrangement of elements that can be divided into equal parts by a plane, axis, or center. In the case of methane, it exhibits several symmetrical characteristics.
Firstly, methane has a tetrahedral molecular geometry, with the carbon atom at the center and four hydrogen atoms positioned around it. This geometry ensures that the molecule is symmetrical in terms of its spatial arrangement.
Each hydrogen atom is located at one of the vertices of the tetrahedron, forming equal angles and distances with respect to the central carbon atom. This symmetry is maintained regardless of the orientation of the molecule.
Additionally, methane possesses rotational symmetry. It can be rotated around any of the carbon-hydrogen bonds, and the molecule will retain its overall appearance.
The symmetry of methane arises from its molecular structure and the equal distribution of electron density around the central carbon atom. The four hydrogen atoms are bonded to the carbon through sigma bonds, which have a cylindrical symmetry. This balanced arrangement of the atoms contributes to the overall symmetry of the molecule.
For more such questions on methane visit:
https://brainly.com/question/25207057
#SPJ8
Which process takes place in the conversion of iron into steel?
Answers
Iron is changed into steel by blowing oxygen through the molten metal from the Blast Furnace. This oxidises the impurities in the molten metal. Carbon is a major impurity in Blast Furnace metal.
It's MISSING PLEASE I need the answer now please asap you will be marked brainiest
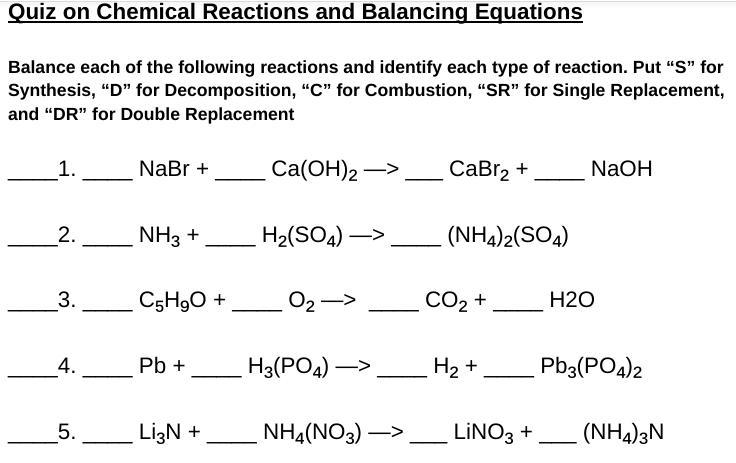
Answers
#1
\(\\ \tt\hookrightarrow 2NaBr+Ca(OH)_2\longrightarrow CaBr_2+2NaOH\)
DR#2
\(\\ \tt\hookrightarrow 2NH_3+H_2SO_4\longrightarrow (NH_4)_2SO_4\)
S#3
\(\\ \tt\hookrightarrow 2C_5H_9O+14O_2\longrightarrow 10CO_2+9H_2O\)
C#4
\(\\ \tt\hookrightarrow 3Pb+2H_3(PO_4)\longrightarrow 3H_2+Pb_3(PO_4)_2\)
SRIf the reaction of 5.87 moles of potassium with excess hydrobromic acid was able to produce 2.49 mol H2, what is the percent yield of hydrogen gas? (3 points)
Unbalanced equation: K + HBr yields KBr + H2
1. 14.6%
2. 29.2%
3. 42.4%
4. 84.8%
Answers
Answer: 2. 29.2%
Explanation:
Suppose 215 g of NO3- flows into a swamp each day. What volume of CO2 would be produced each day at 17.0°C and 1.00 atm?
Answers
Answer:
The answer is "\(41.23 \ L\ N_2\)"
Explanation:
\(2 NO_3^{-} + 10 e^{-} + 12 H^{+} \longrightarrow N_2 + 6 H_2O\\\\= \frac{( 215 \ g \ NO_3^{-})}{(62.0049 \frac{\ g NO_3^{-}}{mol})} \times \frac{(1 \ mol \ N_2}{ 2 \ mol \ NO_3^{-})}\\\\\)
\(=3.46746789 \times 0.5\\\\= 1.733 \ mol \ N_2 \\\\\to V = \frac{nRT}{P} \\\\= (1.733 \ mol) \times (0.08205746 \frac{L\ atm}{Kmol}) \times \frac{ (17 + 273) K}{(1.00 atm)}\\\\= 41.23\)
The volume of CO2 is 206.27 L
The ideal gas equation is used to determine the volume, pressure, temperature, or number of moles. It can be mathematically expressed as:
PV = nRT
From the given information:
The equation for the reaction can be expressed as:
\(\mathbf{2NO_3^-_{(aq)} + 5CO_{(g)} + 2H^+_{(aq)} \to N_2{(g)} + 5CO_2_{(g)} + H_2O_{(l)}}\)
The mass of NO₃⁻ = 215 gThe temperature = 17.0°C = (273 + 17) = 290 KPressure = 1.00 atmThe number of moles of CO2 from the reaction is;
\(\mathbf{= \dfrac{215 \ g}{62.0049} \times \dfrac{5 \ mol \ of \ CO_2}{2 \ mol \ of \ NO_3^-} }\)
\(\mathbf{= 8.669 \ moles \ of \ CO_2 }\)
From ideal gas, by making the volume the subject of the, we have:
The volume of CO₂ \(\mathbf{V= \dfrac{nRT}{P}}\)
\(\mathbf{V= \dfrac{8.669 \ moles \times 0.08205 L atm/ kmol \times 290\ K}{1 \ atm }}\)
\(\mathbf{V= 206.27 \ L \ of \ CO_2 \ gas}\)
Learn more about the volume of CO2 gas here 206.27 L
https://brainly.com/question/14187028?referrer=searchResults
Calculate the relative molecular mass of hydrated Copper (II) tetraoxosulphate (VI) CuSO4.5H₂O (Cu = 64 S = 32 H = 1 0 = 16).
Answers
The relative molecular mass of hydrated Copper (II) tetraoxosulphate (VI) CuSO4.5H₂O is 249.
What is molecular mass?Molecular mass is a measure of the total mass of one mole of a substance, which is defined as the mass of the substance divided by the number of molecules it contains. It is typically expressed in g/mol and is also known as molar mass. Molecular mass is determined by the types and number of atoms that compose a molecule, and is an important factor in understanding the properties of a substance.
This is calculated by adding the atomic masses of all the atoms present in the compound.
The atomic mass of copper is 64, sulphur is 32, oxygen is 16, and hydrogen is 1.
So, the relative molecular mass of hydrated Copper (II) tetraoxosulphate (VI) CuSO4.5H₂O is 64 + 32 + (16*4.5) + (1*5) = 249.
To learn more about molecular mass
https://brainly.com/question/24727597
#SPJ1
thsof
is which acid H2S04
Answers
Explanation:
H2So4=sulphuric acid , strong acid
Shivani measures out 6.0 moles of epsom salt (MgSO) to put her bath. How many grams MgSO4 went in the bath?
Answers
Answer: 720 g
Explanation:
To solve this we must be knowing each and every concept related to mole. Therefore, shivani measures out 6.0 moles of epsom salt (MgSO\(_4\)) to put her bath. 1,566g MgSO\(_4\) went in the bath.
What is mole?A mole is merely a measuring unit. In reality, it is one of its International System of Units' seven foundation units. When current units are insufficient, new units are created.
Chemical reactions frequently occur at levels where employing grams would be inappropriate, but using absolute quantities of atoms/molecules/ions would also be misleading.
For all practical reasons, one mole of a substance in grams is nearly equal to 1 molecule for the compound per daltons.
Mathematically,
number of mole =given mass ÷ molar mass
number of mole=6.0 moles
molar mass of epsom= 261.47g/mol
substituting all the given values in the above equation, we get
6.0 moles =mass of epsom ÷ 261.47g/mol
mass of epsom =1,566g
Therefore, 1,566g MgSO\(_4\) went in the bath.
To know more about mole, here:
brainly.com/question/15209553
#SPJ2
:
1)How many molecules are there in 2.3 grams of NH SO.?
2)How many grams are there in 3.3 x 102 molecules of N..?
3)How many molecules are there in 200 grams of CCI.?
How many grams are there in 1 x 10 molecules of BCI.?
How many grams are there in 4.5 x 10- molecules of Ba(NO)?
How many molecules are there in 9.34 grams of Lici?
How many grams do 4.3 x 10 molecules of UF weigh?
How many molecues are there in 230 grams of N-OH?
Answers
1)How many molecules are
there in 2.3 grams of NH
SO.?
Answer:
1.69 x 10^22
A chemical equation is given
below. How would you classify
this reaction?
Na₂CO3 → Na₂O + CO₂
A.single replacement
B. Synthesis
C. Combustion
D. Decomposition
Answers
D (because because it results in two compounds from one substance)
2.00 g of an unknown gas at STP fills a 500. mL flask. What is the molar mass of the gas?
Answers
The molar mass of the unknown gas in the 500 mL flask at stp is 89.7 g/mol
We'll begin by calculating the number of mole of the gas that occupied 500 mL at stp.
Recall at stp:
22400 mL = 1 mole of the gas
Therefore,
500 mL = 500 / 22400 = 0.0223 mole of gas.
Finally, we shall determine the molar mass of the gas. This can be obtained as follow:
Mass of gas = 2 g
Mole of gas = 0.0223 mole
Molar mass of gas =?Molar mass = mass / mole
Molar mass of gas = 2 / 0.0223
Molar mass of gas = 89.7 g/molTherefore, the molar mass of the unknown gas is 89.7 g/mol
Learn more: https://brainly.com/question/11676583
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
Which of these is the smallest?
atom
molecule
proton
an ant
Answers
Answer
proton
Explanation:
how much 0.100 m h2so4 are needed to make 25.0 ml of 0.00500 m solution

Answers
Answer:
3) 1.25
Explanation:
I had the same question and somehow got it right :)
The new volume will be determined by molarity and which will be 1.25 ml.
What is volume?The volume of an object is just a measurement of how much space it takes up.
What is Molarity?
The molality refers to the moles of a solute in relation to the mass of the solvent, whereas the molarity refers to the moles of a solute in based on the volume of the solution. It can be denoted by M.
The formula of Molarity is
\(M_{1} V_{1} =M_{2}V_{2}\)
where, M is molarity and V is volume.
Calculation of volume.
It is given that,\(M_{1} = 0.1 M, M_{2} =0.0025 M.\)
Now, puts the value of given data in molarity equation.
\(M_{1} V_{1} = M_{2} V_{2}\)
\(V_{1} = M_{2} V_{2} /M_{1}\)
\(V_{1}\) = 0.005×25/0.1 = 1.25 ml
Therefore, the correct answer will be option 3.
To know more about molarity and volume click here.
https://brainly.com/question/20366625.
#SPJ2
Total number of protons and neutrons in the what of an atom
Answers
Answer:
The total number of protons and neutrons is the mass number of an atom.
Explanation:
The mass number is made up of the total protons and neutrons in an atom. The mass number represents the mass of the atom in amu (atomic mass units). Electrons are not included in this representation because their mass is negligible (practically 0). The atomic number is made up of the total protons in an atom.
according to the bohr model of an atom, what happens when an electron moves from the second energy level to the third energy level and then back to the second energy level?
Answers
Answer:
when the atom moves to the third energy level, its energy increases. However, when it goes back to the second energy level its overall energy decreases.
Explanation:
the smallest (or innermost) energy level has the least amount of energy and the largest (or outer most) level needs the most amount of energy. In order for the electron to move from one level to the other, it would need to match the energy of that level.
The change in velocity over a specific amount of time Includes speeding up. slowing down or changing direction. This is known as O speed acceleration changing speed
Answers
Answer:
Acceleration
Explanation:
Acceleration = \(\frac{velocity}{time}\)