A 590.0 g brass candlestick has an initial temperature of 98.0°C. If 21100 J of energy is
removed from the candlestick to lower its temperature to 6.8°C, what is the specific heat of
brass?
Answers
Answer:
specific heat of brass is:
c = 0.412 J/g°C
Explanation:
We can use the formula:
Q = mcΔT
where Q is the amount of heat transferred, m is the mass of the brass candlestick, c is the specific heat of brass, and ΔT is the change in temperature.
We know the following values:
m = 590.0 g
ΔT = 98.0°C - 6.8°C = 91.2°C
Q = -21100 J (negative sign indicates that heat is being removed)
Substituting these values into the formula, we get:
-21100 J = (590.0 g) c (91.2°C)
Solving for c, we get:
c = -21100 J / (590.0 g * 91.2°C)
c = -0.412 J/g°C
Note that the negative sign on the specific heat value indicates that heat is being removed from the system. We can ignore the sign when reporting the final answer, so the specific heat of brass is:
c = 0.412 J/g°C
Related Questions
Choose the best tool for each scenario.
Identifying a trend in temperature change over time:
graph
Measuring the mass of a product of a chemical reaction:
Answers
Answer:
a) Graph
b) Weight balance or gas syringe or upside-down measuring cylinder
Explanation:
a) Identifying a trend in temperature change over time - The best tool for this scenario is to represents the temperature daily, weekly, monthly or annually on graph to interpret the fluctuation in temperature owing to local seasonal changes and weather conditions
b) Measuring the mass of a product of a chemical reaction - If the product is solid or liquid then the balance is used to measure the mass. If the product is a gas, then gas syringe or upside-down measuring cylinder is used.
a. Rub the magnet along the nail or knitting needle. Now try picking up the paper clip or tack. Could your nail pick it up? How many can it pick up at once?
b. Now try to make your magnet stronger by rubbing the magnet along the nail or knitting needle for a longer time. Rub only in one direction, not back and forth. This will increase the number of atoms that line up with the magnet. Try to pick up paper clips or thumbtacks. How many can it pick up now?
Answers
Answer:
ight i'll answer both rq
Explanation:
a. Rub the magnet along the nail or knitting needle. Now try picking up the paper clip or
tack. Could your nail pick it up? How many can it pick up at once?
after rubbing the magnet with the nail, it could pick up 7 small paper clips.
b. Now try to make your magnet stronger by rubbing the magnet along the nail or knitting
needle for a longer time. Rub only in one direction, not back and forth. This will increase
the number of atoms that line up with the magnet. Try to pick up paper clips or
thumbtacks. How many can it pick up now?
after rubbing it a second time but for a longer period, it could pick up around 10, almost lifting the 11th.
Answer:
A. After rubbing the magnet with the nail, it could pick up 7 small paper clips.
B. After rubbing it a second time but for a longer period, it could pick up around 10 small paper clips, almost lifting the 11th one.
Which of the following is the correct formula for cesium sulfide
Answers
Answer:
Cs2S
Explanation:
The chemical formula of cesium sulfide is Cs₂S.
What is the chemical formula?A chemical formula gives information about the chemical proportions of atoms of each element in a chemical compound or molecule. The chemical formula of a compound can be written by utilizing the chemical symbols of elements, numbers (subscripts), and other symbols, such as plus (+) and minus (−) signs, commas, dashes, and brackets.
A chemical formula of a compound consists of not any words but can be represented in certain simple chemical structures. Chemical formulae can be more restricted in power than chemical names and chemical structural formulae. In chemistry, we have two kinds of chemical formulas: one empirical formula and another molecular formula.
The chemical formula of cesium sulfide is Cs₂S which is an inorganic salt. It can be produced by the reaction of cesium and sulfur in solvent THF and also can be produced from cesium hydroxide and hydrogen sulfide.
Learn more about chemical formula, here:
brainly.com/question/11140581
#SPJ2
does a reaction occur when aqueous solutions of zinc acetate and ammonium sulfate are combined? Yes or noIf a reaction does occur, write the net ionic equation. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (). If a box is not needed leave it blank.
Answers
Yes, a reaction does occur when aqueous solutions of zinc acetate and ammonium sulfate are combined. The net ionic equation for the reaction is: 2 Zn(CH₃COO)₂ (aq) + (NH₄)₂SO₄ (aq) → 2 ZnSO₄ (aq) + 2 CH₃COONH₄ (aq).
What is reaction?Reaction is a process in which two or more substances interact to produce one or more new substances. It is a process of transformation of one substance or substances into others by changes in their composition. A reaction can happen in the presence of energy such as heat, light or electricity, or in the absence of energy. The substances that initiate a reaction, called the reactants, are changed into the substances created by the reaction, known as the products. Reactions may occur at different rates and may involve different steps, such as complex mechanisms and intermediate compounds. Examples of reactions include combustion, acid-base reactions, oxidation-reduction reactions, and nuclear reactions.
To learn more about reaction
https://brainly.com/question/25769000
#SPJ1
What is the ratio of hydrogen atoms to carbon atoms in a carbohydrate?
Answers
The ratios are 1:2:1. The ratio of carbon, hydrogen, and oxygen in most carbohydrates is 1:2:1.
This means for every one carbon atom and there are two hydrogen atoms and one oxygen atom. It forms the structure of even the most complex carbohydrates such as starch and glycogen. Carbohydrates are the organic molecules madeup of carbon, hydrogen, and oxygen atoms.
The ratio of these three atoms in carbohydrates is most commonly 1:2:1, meaning for every carbon atom, there are two hydrogen atoms and a oxygen atom. This ratio is the same for both monosaccharides and polysaccharides. Monosaccharides are the simplest carbohydrates and are composed of single sugar molecules, while polysaccharides are larger molecules composed of multiple sugar molecules linked together.
To know more about carbohydrates:
brainly.com/question/20290845
#SPJ4
1kg of hydrogen is burned in oxygen how much energy is released
Answers
Answer:
121 million joules energy is released
Explain :
As there are 500 moles of hydrogen gas in a kilogram, this means that burning a kilogram of hydrogen gas releases 500 times as much energy or 121 million joules .
What the anode , cathode and the electrolyte of a cell tha t you might use to electrolyte a spoon made from iron with silver?
Answers
The silver coating on the spoon is produced. When electrolyzing a spoon made from iron with silver, the anode, cathode, and electrolyte that can be used are as follows:
Anode: The anode is a negatively charged electrode, usually made of metal or graphite, that releases electrons during electrolysis. It is made of pure silver.Cathode: The cathode is a positively charged electrode that receives electrons during electrolysis. It is made of iron.Electrolyte: The electrolyte is a solution that conducts electricity and contains ions that can be reduced or oxidized. The electrolyte used for this process is a solution of silver nitrate (AgNO3) in water.The silver ion (Ag+) moves from the anode to the cathode through the electrolyte. At the cathode, it accepts an electron, reducing it to metallic silver (Ag). Fe(s) is oxidized to Fe2+(aq) ion at the anode, while Ag+ ions are reduced to Ag(s) at the cathode. Therefore, the silver coating on the spoon is produced.For such more questions on silver coating
https://brainly.com/question/29736740
#SPJ8
5. One of Dr. Birdley's boxes has a
volume of 120 cm' and a mass of 2400 g.
The density of the rock is
a) 20 g/cm
b) 2520 g/cm
c) 240,480 g/cm
d) 2280 g/cm
Answers
Answer:
a.20g/cm
hope this helps :)
Explanation:
solve with steps plz

Answers
I think it's b
let's work out empirical formula:
CO2 + H20 is CH2O3
the mass of CH2O3 is 12+2+(16×3)=62
work out mass of CO2
mass=number of moles × relative formula mass
mass=1.5 mol × 44 = 66
work out mass of H20
mass=number of moles × relative formula mass
mass=2 × 18 = 36
so the molecular formula is 66+36=102
to calculate simplest molecular formula:
molecular formula mass/empirical formula mass
102/62=2 (rounded to nearest whole number)
back to the empirical formula which is CH2O3
multiply each element by 2 which is
C2H4O6
answer:C2H4
not 100% sure though, but I hope it's been of some use to you:)
Mass of CO_2
\(\\ \sf\longmapsto No\:of\:moles(Molar\:mass)\)
\(\\ \sf\longmapsto 44(1.5)\)
\(\\ \sf\longmapsto 66g\)
And
Molar mass of water=18g/molNo of moles=2Mass of H_2O
\(\\ \sf\longmapsto 2(18)=36g\)
Total mass of products
\(\\ \sf\longmapsto 66+36=102g\)
EMPIRICAL FORMULA:-CH_2O_3
Empirical formula mass:-\(\\ \sf\longmapsto 12+2(1)+3(16)=12+2+48=62g\)
We need n now
\(\\ \sf\longmapsto n=\dfrac{Molecular\:mass\:of\; products}{Empirical\: formula\:mass}\)
\(\\ \sf\longmapsto n=\dfrac{102}{62}\)
\(\\ \sf\longmapsto n=1.64\approx 2\)
Now
\(\\ \sf\longmapsto Molecular\: formula=n(Empirical\: formula)\)
\(\\ \sf\longmapsto 2(CH_2O_3)\)
\(\\ \sf\longmapsto C_2H_4O_6\)
It's Ethene Oxide .
Answer is Ethene(C_2H_4)
Option B is correct
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
Which ingredient is found stronger of the two most commonly used chemical relaxers? A. Bisulfate B. Potassium C. Sodium hydroxide. D. Hydrogen dioxide
Answers
The strongest ingredient of the two most commonly used chemical relaxers is Sodium hydroxide. This ingredient is often found in lye relaxers, which are known for being the most powerful type of relaxer on the market.
Sodium hydroxide has a high pH level and breaks down the protein bonds in the hair, which allows the hair to be reshaped and straightened. Bisulfate and potassium are also commonly used in relaxers, but they are not as strong as Sodium hydroxide. Bisulfate is often found in no-lye relaxers, which are less harsh on the hair and scalp, but also less effective at straightening hair. Potassium is another ingredient found in some relaxers, but it is not typically used as the main active ingredient.
Hydrogen dioxide is not typically found in relaxers at all, as it is a bleaching agent rather than a straightening agent. Overall, when it comes to choosing a relaxer, it is important to consider the strength of the active ingredient as well as the potential risks and benefits of each type of relaxer.
learn more about Sodium hydroxide here: brainly.com/question/29327783
#SPJ11
Rank each of the bonds identified in order of increasing wavenumber: Hint : Stronger bonds (triple bonds > double bonds single bonds) vibrate at higher frequencies:
Answers
The order of increasing wavenumber for the bonds is: single bonds < double bonds < triple bonds. This reflects the relative strengths of the bonds, with triple bonds being the strongest and single bonds being the weakest.
The wavenumber of a bond in a molecule is directly proportional to the frequency of its vibration. Stronger bonds vibrate at higher frequencies, and weaker bonds vibrate at lower frequencies.
Using this information, we can rank the bonds identified in order of increasing wavenumber as follows:
1. Single bonds: These bonds are the weakest and vibrate at the lowest frequency, so they have the lowest wavenumber.
2. Double bonds: These bonds are stronger than single bonds and vibrate at a higher frequency, so they have a higher wavenumber.
3. Triple bonds: These bonds are the strongest and vibrate at the highest frequency, so they have the highest wavenumber.
Therefore, the order of increasing wavenumber for the bonds is single bonds < double bonds < triple bonds. This order reflects the relative strengths of the bonds, with triple bonds being the strongest and single bonds being the weakest.
To know more about wavenumber refer here:
https://brainly.com/question/31452434#
#SPJ11
What are characteristics of reliable science?
A. Bias and controlled variables .
B. Controlled variables and small test groups.
C. Empirical data and bias .
D. Replicable results and empirical data .
Answers
Answer:
Replicable results and empirical data.
Explanation:
Answer:
D
Explanation:
Replicable results mean that the question or test can be replicated to prove the subject in manner. Empirical data means that the question or test can be resolved with the same data, so for example, if a chemist discovers a new chemical equation, they need to write down the formula.
How is matter conserved in the nutrient cycle
Answers
Answer:
matter is conserved because atoms are conserved in physical and chemical process
Explanation:
energy takes different forms. the transfer of energy drives the motion of matter.
Important question, please answer(worth 64 points.)
Balance this equation
___C8H18 + ____O2 ⇒ ___CO2 + ___H2O
Answers
Answer:
Is is talking about h20 water?
Explanation:
Answer:
2C8H18 + 25O2 ----> 16CO2 + 18H2O
Explanation:
Basically you can first list all your elements from your products and reactants:
C 8 x 2 C 1 x 16
H 18 x 2 H 2 x 18
O 2 x 25 O (2 x 18) + (2 x 16)
Be sure to answer all parts. Write the number of individualions per formula unit and the coordination number of themetal ion in each of the compounds below. Dibromobis(ethylcuediamine)cobalt(III) sulfate (Ions? and Coordination number?)
Answers
The compound dibromobis(ethylenediamine)cobalt(III) sulfate, there are 3 individual ions per formula unit: one cobalt(III) complex ion [Co(en)2Br2]+3, one sulfate ion (SO4)^2-, and three water molecules as each formula unit contains three waters of hydration.
For Dibromobis(ethylcuediamine)cobalt(III) sulfate, the number of individual ions per formula unit and the coordination number of the metal ion are: - Number of individual ions per formula unit: There are a total of 6 ions per formula unit.
The compound has the following formula: [Co(ethylenediamine)2Br2]SO4. This means that there are two ethylenediamine ligands, each contributing 2 nitrogen atoms for a total of 4 nitrogen atoms.
Each nitrogen atom has a lone pair of electrons that can coordinate with the cobalt ion. There are also 2 bromide ions and 1 sulfate ion in the formula.
So, the total number of individual ions per formula unit is 4 nitrogen atoms + 2 bromide ions + 1 sulfate ion = 6 ions. - Coordination number of the metal ion: The metal ion in this compound is cobalt(III). Cobalt(III) has a coordination number of 6, which means that it can coordinate with 6 ligands. In this compound, there are 2 ethylenediamine ligands, each contributing 2 nitrogen atoms for a total of 4 nitrogen atoms.
The coordination number of the metal ion (cobalt) in this compound is 6, as there are two ethylenediamine (en) ligands, each with two nitrogen atoms coordinating to cobalt, and two bromine atoms also coordinating to cobalt (2x2 + 2 = 6).
The 4 nitrogen atoms coordinate with the cobalt ion, leaving 2 open coordination sites. These sites are occupied by the 2 bromide ions, giving a coordination number of 6 for the cobalt ion.
Visit here to learn more about Atoms:
brainly.com/question/26952570
#SPJ11
PLEASE HELP ME ASAP!!! 10 POINTS!!
The three equations below represent nuclear fission and nuclear fusion reactions. For each equation, tell whether fission or fusion has occurred and write the missing term in the equation.
A) ²³⁵₉₂ U+n→⁹⁵₃₈Sr+____+2n
B) ³₁H+²₁H→⁴₂H+____
C) ____+n→⁹²₃₉Y+¹⁴⁰₅₃I+2n
Answers
Explanation:
A) fission: Iodine-140 (atomic number = 53)
B) fusion: 1 neutron
C) fission: Uranium-233 (atomic number = 92)
What volume of CO2 forms when 8.00 L of hexane burn, assuming the two volumes are measured under the same conditions? What volume of oxygen will be needed?
pls help asap
Answers
Answer:
48.00L of CO₂ are formed.
76.00L of O₂ are needed
Explanation:
Based on the reaction:
C₆H₁₄ + 19/2O₂ → 6CO₂ + 7H₂O
Where 1 mole of hexane reacts with 19/2 of O₂ to produce 6 moles of CO₂ and 7 moles of H₂O
Using Avogadro's law, volume is directely proportional to moles of gas.
If 1 mole of reaction of hexane is equal to 8.00L, 6 moles of CO₂ that are produced have as volume:
8.00L ₓ 6 =
48.00L of CO₂ are formed.Again, for a complete burning of hexane, you need 19/2 times of moles of oxygen. As you have 8.00L of hexane, you need:
8.00L × (19/2) =
76.00L of O₂ are neededCalculate the amount in grams of solid K2CrO4 to make 100ml of a0.2 M aqueous solution of K2CrO4.(Please explain SF)
Answers
The first step is to convert the volume of solution in mililiters to liters, use a conversion factor to do it:
\(100mL\cdot\frac{1L}{1000mL}=0.1mL\)Now, multiply the volume of the solution by its concentration to find the number of moles that are needed to make that solution (remember that M=mol/L):
\(0.1L\cdot\frac{0.2mol}{L}=0.02mol\)Finally, convert this amount of moles to grams using the molecular weight of K2CrO4 (MW=194.2g/mol):
\(0.02mol\cdot\frac{194.2g}{mol}=3.884g\)The amount of grams of K2CrO4 to make 100ml of solution is 3.884g.
Predict whether the bond between each pair of atoms will be no polar convalent, polar covalent or ionic
A. Carbon and fluorine
B. Iron and Oxygen
C. Chlorine and chlorine.
PLEASE ITS A TWST QUESTION WORTH 5 marks.
PLEASEEEE HELPPP
Answers
Which of the following statements regarding the movement of electrons during cellular respiration is true?
A. Electrons tend to move away from O2.
B. O2 is reduced when it accepts electrons and forms water.
C. The electrons release large amounts of energy each time they are transferred from one molecule to another.
D. O2 is eventually oxidized by the electrons to form water.
Answers
Answer:
B. O2 is reduced when it accepts electrons and forms water.
Explanation:
During cellular respiration water is formed when oxygen receives electrons and picks up protons at the conclusion of the electron transport chain. The electron transport chain will stop functioning if there isn't enough oxygen to receive electrons (for example, because a person isn't breathing in enough oxygen), and ATP will no longer be created by chemiosmosis. Cells can't carry out the reactions they need to function if they don't even have enough ATP, and they may perish after a long period of time.
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
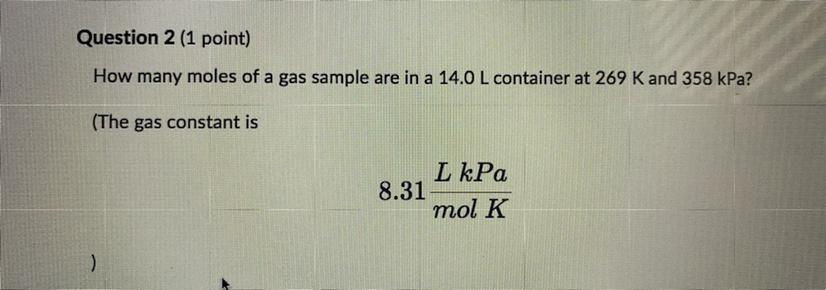
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
You poured out a little (or a lot) too much of a chemical solution. it is ok to pour it back into the bottle.
a) true
b) false
Answers
(b) false
It is not ok to pour it back in the bottle
Once a chemical is taken outside its bottle or container , it reacts with various gases present in the natural environment and also with those chemical which are being released in that laboratory.This will become a source of possible contamination for the entire contents of the stock bottle.The disposal of entire chemicals should be done as per instructed on the reagent bottle to prevent any dangerYou should never put the used spatula inside the reagent bottle.Do not put the excess chemicals inside the sink also , dispose it as it was instructed on the reagent bottle.To know more about chemical safety please refer:
https://brainly.com/question/4430099
#SPJ4
The temperature of 15 grams of water is increased by 3.0 Celsius degrees. How much heat was absorbed by the water? (specific heat of water: 4.18 J/g deg C)
Answers
Answer:
188.1J
Explanation:
obtained from Q=MCT
Why do ionic compounds have higher electrical conductivity than molecular compounds?
support your answer with an example.
Answers
Answer:
Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. Covalent compounds have bonds where electrons are shared between atoms.
A student is building an electrical circuit.
Which material should she choose for the wires, and why?
O A. A metal such as copper, because its atoms have very mobile
electrons
O B. A metalloid such as germanium, because only some of its atoms
have mobile electrons
O c. A noble gas such as krypton, because its atoms are far apart and
it is nearly inert
O D. A nonmetal such as carbon, because its atoms have tightly bound
electrons
Answers
Answer:
A a metal such as copper
Explanation:
Answer:
A metal such as copper, because its atoms have very mobile electrons
Explanation:
Describe the main energy level shape and of the 2p * orbital
Answers
Answer:
The letters, s, p, d, and f designate the shape of the orbital. (The shape is a consequence of the magnitude of the electron's angular momentum, resulting from its angular motion.) An s orbital is spherical with its centre at the nucleus.
Which of the following statements correctly describe the vsepr model?
a. According to this model, the valence electrons around a central atom are located as far from each other as possible. b. A Lewis structure is necessary in order to apply the VSEPR model. c. The VSEPR model states that the electron domains surrounding a central atom attract each other. d. The VSEPR model is used to predict the number of bonds a given element will form. e. The VSEPR model is used to predict the geometry of a covalently bonded species
Answers
The correct options to the question are option A, option B and option E that is According to this model, the valence electrons around a central atom are located as far from each other as possible, A Lewis structure is necessary in order to apply the VSEPR model and The VSEPR model is used to predict the geometry of a covalently bonded species.
The VSEPR theory or Valence Shell Electron Pair Repulsion Theory is the theory given to explain the repulsion of electrons present in the valence shell of an atom. This theory is used to predict the shape of the molecules with the help of the electrons present in the valence shell. This theory is based on the fact that the shape taken by the molecule will be in such a manner that the repulsion of the electrons present in the valence shell of an atom is minimum. Other postulates of this theory include that according to this model, the valence electrons around a central atom are located as far from each other as possible.Lewis's structure is necessary in order to apply the VSEPR model and the VSEPR model is used to predict the geometry of a covalently bonded species.Learn more about VSEPR theory at:
brainly.com/question/14992767
#SPJ4
which group iia metal magnesium or calcium is more active
Answers
Magnesium (Mg) is more active than calcium (Ca) in Group IIA of the periodic table.The Group IIA metal that is more active between calcium and magnesium is magnesium (Mg).
Magnesium is a chemical element with the atomic number of 12 and symbol Mg. It belongs to the Group IIA alkaline earth metals in the periodic table.
Calcium and magnesium are two of the five elements in Group IIA of the periodic table that have the most outstanding chemical properties that are critical to life.Magnesium has a strong reducing effect.
Calcium is less active than magnesium because it is harder to reduce its noble gas configuration to 0, making it less electropositive and less reactive.
Magnesium, on the other hand, has a smaller radius than calcium and is more electronegative, allowing it to lose two electrons to form an Mg2+ cation with ease.
Therefore, magnesium (Mg) is more active than calcium (Ca) in Group IIA of the periodic table.
To know more about Magnesium visit:
https://brainly.com/question/8351050
#SPJ11
give the number of carbon atoms hydrogen atoms and fluorine atoms in a molecule of hcfc-22
Answers
The number of carbon atoms hydrogen atoms and fluorine atoms in a molecule of hcfc-22 is one.
HCFC-22 has one carbon atom, two hydrogen atoms, one fluorine atom, and three chlorine atoms in a single molecule. It has a molecular formula of CHClF2. It is an organohalogen compound that is used as a refrigerant and is also known as chlorodifluoromethane.The molecular formula of HCFC-22 is CHClF2.
It has one carbon atom, two hydrogen atoms, one fluorine atom, and three chlorine atoms. Therefore, the number of carbon atoms in a molecule of HCFC-22 is 1, the number of hydrogen atoms is 2, and the number of fluorine atoms is 1.
HCFC-22 has one carbon atom, two hydrogen atoms, one fluorine atom, and three chlorine atoms in a single molecule with the molecular formula CHClF2.
Learn more about carbon -
brainly.com/question/27860158
#SPJ11