A 51.4g sample of glass is put into a calorimeter (see sketch at right) that contains 300.0g of water. The glass sample starts off at 98.5°C and the temperature of the water starts off at 20.0°C. When the temperature of the water stops changing it's 22.1°C. The pressure remains constant at 1atm.
Calculate the specific heat capacity of glass according to this experiment. Be sure your answer is rounded to the correct number of significant digits.
Answers
The specific heat capacity of glass is 0.84 J/g°C.
To calculate the specific heat capacity of glass, we can use the formula:
q = m × c × ΔT
Where q is the amount of heat transferred, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
In this experiment, the heat transferred from the glass to the water is equal to the heat gained by the water:
q(glass) = -q(water)
We can rearrange the formula to solve for the specific heat capacity of glass:
c = -q(water) / (m(glass) × ΔT)
Plugging in the values, we get:
c = - (300.0g) × (4.18 J/g°C) × (22.1°C - 20.0°C) / (51.4g × (98.5°C - 22.1°C))
Simplifying the equation gives us:
c = 0.84 J/g°C
The specific heat capacity of glass is 0.84 J/g°C according to this experiment.
To know more about specific heat capacity, visit:
https://brainly.com/question/29766819
#SPJ11
Related Questions
How many significant figures does 0.062400 have?
Answers
what does a lineweaver-Burk plot look like?
Answers
The lineweaver-Burk plot is applicable for enzyme kinetics and image of same is attached below.
Enzymes are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life.
When used for determining the type of enzyme inhibition, the Lineweaver–Burk plot can between distinguish competitive, pure non-competitive and uncompetitive inhibitors. The various modes of inhibition can be compared to the uninhibited reaction.
Learn more about enzymes,here:
https://brainly.com/question/31385011
#SPJ4

experiment 1: calculate the combined mass of the two reactants: hydrochloric acid and sodium hydroxide
Answers
The combined mass of hydrochloric acid and sodium hydroxide is determined by adding their individual masses.
When calculating the combined mass of hydrochloric acid and sodium hydroxide, we need to consider the individual masses of these two substances. Hydrochloric acid (HCl) has a molecular formula of HCl and consists of one hydrogen atom (H) and one chlorine atom (Cl). Sodium hydroxide (NaOH), on the other hand, is composed of one sodium atom (Na), one oxygen atom (O), and one hydrogen atom (H). To calculate the combined mass, we add the individual masses of these reactants.
The molar mass of hydrogen (H) is approximately 1 gram/mol, while the molar mass of chlorine (Cl) is approximately 35.5 grams/mol. Sodium (Na) has a molar mass of around 23 grams/mol, oxygen (O) has a molar mass of approximately 16 grams/mol, and hydrogen (H) has a molar mass of around 1 gram/mol.
To determine the combined mass of hydrochloric acid and sodium hydroxide, we multiply the number of atoms of each element by their respective molar masses and sum them up. For example, hydrochloric acid has one hydrogen atom and one chlorine atom, so the total mass would be 1 gram/mol (hydrogen) + 35.5 grams/mol (chlorine). Similarly, sodium hydroxide has one sodium atom, one oxygen atom, and one hydrogen atom, resulting in a combined mass of 23 grams/mol (sodium) + 16 grams/mol (oxygen) + 1 gram/mol (hydrogen).
Learn more about hydrochloric acid
https://brainly.com/question/1451933
#SPJ11
What is the frequency of a red laser that has a wavelength of 676 mn
Answers
The frequency of a red laser that has a wavelength of 676 nm would be 4.43 x \(10^{14\) hertz.
Frequency of wavesThe frequency and wavelength of a wave are related by the following equation:
λf = c
Where λ is the wavelength of the wave in meters, f is the frequency in Hertz, and c is the speed of light in a vacuum.
in this case, λ = 676 nm = 6.76 x \(10^{-7\) m
c = 299,792,458 m/s
Making f the subject of the formula:
f = c/λ
= 299,792,458/6.76 x \(10^{-7\)
= 4.43 x \(10^{14\) hertz
In other words, the frequency of a red laser that has a wavelength of 676 nm would be 4.43 x \(10^{14\) hertz.
More on waves can be found here: https://brainly.com/question/29334933
#SPJ1
what is the product of aluminum +zinc sulfate solution
Answers
Answer:
ZINC ALUMINATE
Looking at the chemical formulae of these compounds, which is an alkane? * 1 point C3H8 C2H4 C2H5OH C5H10
Answers
Answer:
(C3H8 )
Explanation:
Alkanes have the general formula of CnH2n+2 where n is the number of carbon atoms
.Which of the following is a characteristic of both scientific theories and laws?Immersive Reader
(3 Points)
not subject to change
based on a scientist's opinion
explains natural phenomena
based on scientific evidence
Answers
Answer:
its B
Explanation:i took the test
Answer:
I believe the answer is ; explains natural phenomena.
Explanation:
How do the force factors compare to the products of the masses?
Answers
Answer:
The force factors are equal to the first numbers of the products of the masses in scientific notation.
Explanation:
Ex. 1.0 x 10^5 kg^2 then the force factor would be 1.
ex. 3.0 x 10^12 kg^2 then the force factor would be 3.
To solve this we must be knowing each and every concept related to scientific notation and its calculations. Therefore, in scientific notation, the force factors seem to be equivalent to the first digits of the masses' products.
What is scientific notation?Mathematics is a way of representing numbers using a decimal number ranging from one to 10, but not 10 multiplied by a factor of 10. The most common type of mathematical notation is All numbers in scientific notation are expressed in the generic form N 10m.
In scientific notation, the force factors seem to be equivalent to the first digits of the masses' products.
Example1 1.0 x 10⁵ kg² , force factor is 1.
Example2 3.0 x 10¹² kg² ,force factor is 3.
Therefore, in scientific notation, the force factors seem to be equivalent to the first digits of the masses' products.
To learn more about scientific notation, here:
https://brainly.com/question/18073768
#SPJ2
which of the following minerals crystallize early in bowen's reaction series? 1. mafic minerals 2. quartz 3. muscovite 4. potassium feldspar
Answers
The minerals that crystallize early in Bowen's reaction series are the mafic minerals.
These minerals, such as olivine and pyroxene, have a higher melting point and are the first to form as magma cools. As the magma continues to cool, minerals with lower melting points, such as feldspar and quartz, begin to crystallize. Muscovite and potassium feldspar are both part of the group of minerals that form later in the reaction series. The order of crystallization in Bowen's reaction series is important in understanding how rocks form and the different mineral compositions that result. In summary, mafic minerals are the first to crystallize, followed by intermediate and felsic minerals as the magma cools.
to know more about Bowen's reaction series visit:
https://brainly.com/question/4860542
#SPJ11
28 g sodium nitrate is dissolved in water to make 500 g of solution. what is the percent sodium nitrate in the solution?
Answers
The percent sodium nitrate in the solution Molarity, molality, or % can be used to express a substance's concentration.
What is sodium nitrate?The chemical substance with the formula Nano 3 is sodium nitrate. As distinguish it from common saltpeter, potassium nitrate, this alkali metal nitrate salt is also referred to as Chile saltpeter. Nitratine, nitratite, and soda niter are further names for the mineral form. Headaches, nausea, vomiting, diarrhea, and stomach pain are all possible side effects of sodium nitrite. For sodium nitrite, no occupational exposure limits have been set. Although sodium nitrite is a good preservative, it also has significant disadvantages. The fact that it can produce nitrosamines, which cause cancer, is the most serious worry. When sodium nitrite interacts with particular meat proteins, these nitrosamines can result. Foods have long been preserved with salts like sodium nitrate.To learn more about sodium nitrate refer to:
https://brainly.com/question/29614071
#SPJ4
Earth is removed from the top to reveal the coal. Which type of coal mine does this describe? undersea subterranean drill strip
Answers
Answer:
Strip
Explanation:
Strip mining removes Earth in long strips from the top to reveal the coal. This earth to be removed is known as 'overburden'. The overburden from the first strip is referred to as out-of-pit dumping. Strip mining is a part of surface mining.
Strip mining destroys forests, landscapes, forests and wildlife habitats as trees and plants are removed from the mining area.
Therefore,
this describes the Strip coal mine.
Answer:
strip Mining.
Explanation:
I have four questions:
1- what can you say about the number of protons in these three metals as you look down in the group. (lithium, sodium, and potassium.)
2- what can you say about the mass number of the three metals.(lithium, sodium, and potassium.)
3- what is similar about the structures of an atom of lithium and an atom of sodium?
4- what is similar about the structures of lithium sodium and potassium?
Answers
The number of protons in lithium is 3, in sodium is 11 and in potassium is 19 whereas the mass number of lithium is 6.941 u, the mass number of sodium is 22.989769 u and the mass number of potassium is 39.0983 u.
What is the mass number of lithium, sodium, and potassium?Numbers of Protons:
The total number of protons in lithium is 3.
The total number of protons in sodium is 11.
The total number of protons in potassium is 19.
Mass Number:
The mass number of lithium is 6.941 u.
The mass number of sodium is 22.989769 u.
The mass number of potassium is 39.0983 u.
Structure of Lithium and Potassium:
Lithium and potassium have a similarity in them that they all have one electron in their outermost orbit.
Structure of Sodium and Potassium:
Sodium and potassium have a similarity in them that they all have one electron in their outermost orbit.
So we can conclude that the number of protons in lithium is 3, in sodium is 11 and in potassium is 19.
Learn more about Proton here: https://brainly.com/question/1805828
#SPJ1
How will the concentration of lactose be measured and what assumption must be made?
Answers
To measure the concentration of lactose, one can use methods such as the enzymatic assay or high-performance liquid chromatography (HPLC). The assumption that must be made is that other sugars or compounds present in the sample do not interfere with the measurement.
The concentration of lactose can be measured using various methods such as enzymatic assays or high-performance liquid chromatography (HPLC). However, an assumption that must be made when measuring the concentration of lactose is that there are no interfering substances present that could interfere with the accuracy of the measurement. It is also important to ensure that the sample is properly prepared and the measurements are taken under controlled conditions to ensure the accuracy of the results.
Learn more about high-performance liquid chromatography here:
https://brainly.com/question/31046657
#SPJ11
Find the height of a triangle that has an area of 30 square units and a base measuring 12 units.
3 units
5 units
8 units
9 units
Answers
a copper ore contains 3.00% of copper carbonate, CuCO3, by mass. Which mass of copper would be obtained from 1 tonne of the ore?
A 1.91kg B 3.71kg C 15.3kg D 58.4kg
Answers
Answer:
(c) 15.39 kg of copper present in 1tonne of ore.
Explanation:
We are given that ore contains 3% of copper carbonate
1tonne = 1000kg
3% of copper carbonate in 1000kg of ore will be
= \(\frac{3}{100}*1000\) = 30kg
30 kg of copper carbonate is present in ore
CuCO3 has 63.5g of cupper present in it
molar mass of CuCO3 = 123.5
so the percentage of copper present in CuCO3
= \(\frac{63.5}{123.5}*100\) = 51.3% of copper present per kg CuCO3
Now
amount of copper present in 30kg of CuCO3
= \(\frac{51.3}{100}* 30\) = 15.39kg
15.39 kg of copper present in 1tonne of ore
The mass of copper obtained from 1 tonne of the ore is 15.4 kg
The copper ore has 3% of copper carbonate by mass.
The mass of copper carbonate in 1 tonne of the ore can be calculated below.
1000 kg = 1 tonne
Therefore,
mass of copper carbonate = 3 /100 × 1000 = 30 kg
atomic mass of copper = 63.5 g
molar mass of CuCO₃ = 123.55 g
123.55 g of CuCO₃ gives 63.5 g of copper
30, 000g of CuCO₃ will give ? of copper
cross multiply
mass of copper = 30,000 × 63.5 / 123.55
mass of copper = 1905000 / 123.55 = 15418.8587616 g
mass of copper = 15418.8587616 / 1000 ≈ 15. 4 kg
read more: https://brainly.com/question/1237330?referrer=searchResults
A pi bond is the result of the a) overlap of two s orbitals. b) overlap of an s orbital and a p orbital. c) overlap of two p orbitals along their axes. d) sideways overlap of two parallel p orbitals. e) sideways overlap of two s orbitals.
Answers
A pi bond is the result of the d) sideways overlap of two parallel p orbitals.
Pi bonds are bonds that occur as a result of overlapping orbitals of atoms that are not in the bond axis. Each p orbital that contributes to a pi bond has two lobes and has a node at the core.
The pi orbital can hold a maximum of two pairs of electrons. Whereas each electron in a pi bond is also called a pi electron, the pi electrons are used for double bonds or triple bonds. The 2p orbital of carbon has slightly higher energy than the sp2 orbital, so the pi bond formed from two 2p orbitals has somewhat higher energy and is slightly less stable than the sp2-sp2 sigma bond.
Learn more about pi bonds at:
https://brainly.com/question/13243902
#SPJ4
Which of the following pictures correctly shows the structure of hydronium ion?
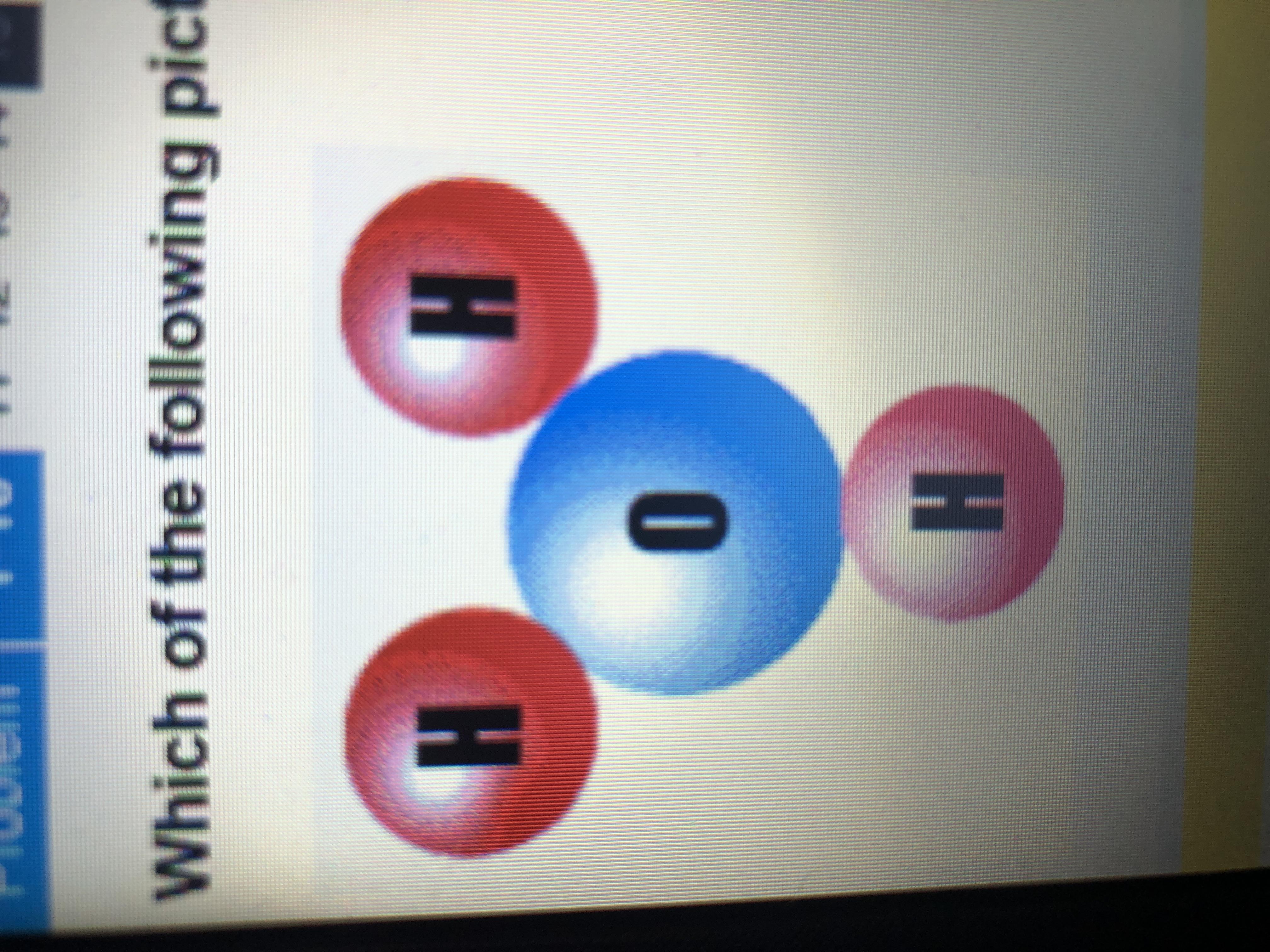



Answers
Answer: The first picture
Explanation:
The structure of hydronium ion is correctly represented in the first structure where there are three hydrogen atoms and one oxygen atom.
What is an ion?An ion is defined as an atom or a molecule which has a net electrical charge. There are 2 types of ions :1) cation 2) anion . The cation is the positively charged ion and anion is the negatively charged ion . As they are oppositely charged they attract each other resulting in the formation of ionic bond.
Ions consisting of single atom are mono-atomic ions while which consists of two or more ions are called as poly-atomic ions . They are created by chemical interactions . They are very reactive in their gaseous state and rapidly react with oppositely charged ions resulting in neutral molecules.
Learn more about ion,here:
https://brainly.com/question/1488567
#SPJ2
What is the definition of Monohybrid cross
Answers
Answer:
A monohybrid cross is a cross between two organisms with different variations at one genetic locus of interest. The character being studied in a monohybrid cross are governed by two or multiple variations for a single locus.
Explanation:
A monohybrid cross is a cross between two organisms with different variations at one genetic locus of interest. The character being studied in a monohybrid cross are governed by two or multiple variations for a single locus.
Answer:
a monohybrid crosses a cross between two organisms with different variations at one genetic locus of interest
Suppose that, in an alternate universe, the possible values of ml were the integer values including 0 ranging from −l−1 to l+1 (instead of simply −l to +l). How many orbitals would exist in each of the following sublevels?
s sublevel
p sublevel
d sublevel
Answers
The orbitals would exist in each of the following sublevels are:
s sublevel = 3 orbitals
p sublevel = 5 orbitals
d sublevel = 7 orbitals
if ml range to -l -1 to +l +1
for s sublevel : l = 0
ml = -1 , 0, +1
therefore, s sublevel have 3 orbitals
for p sublevel : l = 1
ml = -2, -1. 0, +1, +2
therefore, p sublevel have 5 orbital
for d sublevel : l = 2
ml = -3, -2, -1, 0, +1, +2, +3
therefore, d sublevel have 7 orbital.
Thus, s sublevel have 3, p sublevel have 5, d sublevel have 7 orbitals.
To learn more about sublevel here
https://brainly.com/question/14650726
#SPJ4
A gas mixture is made from 15.6 g of bromine gas and 13.8 g of chlorine gas. The total pressure of the mixture is 0.555 atm. What is the partial pressure of the bromine gas?
Answers
Answer:
the partial pressure of bromine gas is 0.186 atm
Explanation:
If alkali A has a pH value of 13 and alkali B has a pH value of 9, explain which is most likely to be used in indigestion tablets as a remedy for excess stomach acid. *
Answers
Answer:
Alkalai B
Explanation:
B because its a weak alkali ie baking soda ph 8.3(sodium bicarbonate)which is used as a remeady of heartburns and stomach acids.
In the reaction below green chlorine gas and brown
iodine chloride forms yellow iodine trichloride
Cl2 (g) + ICI () = IC13 (s)
If some chlorine is removed from the reaction, how will
it affect the color of the mixture?
A. It will become more green.
B. It will become more yellow.
C. It will become more brown.
OD. It will not change.
Answers
Answer:
The correct option is;
C. It will become more brown
Explanation:
Given that the reaction is as follows;
Cl₂(g) + ICl (g) ⇄ ICl₃
We have that the reaction and a decrease in the concentration of the reactants will favor the reverse reaction that is the decomposition of the yellow iodine trichloride and the formation of green chlorine gas and brown iodine chloride
Given that some green chlorine gas, which is part of the reactant, will be removed, the reverse reaction will be favored and initial concentration of the yellow iodine trichloride and the green chlorine gas will be reduced while the proportional concentration of the brown iodine chloride will increase and the mixture will become more brown.
Answer:
become more green
Explanation:
5.4 g of Aluminium reacts with 300 mL of 0.2 mol/L hydrochloric acid solution. A. Write equation for the reaction taking place. B. Specify which reactant is limiting and which reactant is excess. C. Find volume of the gas collected at S.T.P d. How many grams of salt are produced at the end of the reaction? E. How many grams of the excess reactant are left ate the end of the reaction? Given: Al=27 , H=1 , Cl=35.5 Chemistry grade 10
Answers
Answer:
A. 2 Al (s) + 6HCl (aq) → 2AlCl₃ (s) ↓ + 3H₂ (g)
B. Al is the excess reactant and HCl is the limiting.
C. 0.672 L of H₂ produced at STP
D. 2.67 g of AlCl₃ are made in this reaction.
E. 4.86 g of Al remain after the reaction goes complete.
Explanation:
We star from the reaction:
2 Al (s) + 6HCl (aq) → 2AlCl₃ (s) ↓ + 3H₂ (g)
2 moles of aluminum, react with 6 moles of HCl in order to produce 2 moles of aluminum chloride and 3 mol of H₂ gas.
We determine moles of each reactant:
[HCl] = 0.2M → 0.2 mol/L . 0.3L = 0.060 moles
(we converted 300 mL to 0.3L)
5.4 g of Al . 1mol / 26.98g = 0.200 moles
Ratio is 2:6 (3). 2 mol of Al react to 6 mol of HCl
0.2 moles of Al may react with (0.2 . 6) /2 = 0.6 mol of acid
We have 0.06 moles, and we need 0.6 mol of acid, so the HCl is the limiting reactant. Then, the Al is the excess:
6 moles of HCl need 2 moles of Al to react
Then 0.06 moles of HCl will react to (0.06 . 2) /6 = 0.02 moles
If we have 0.2 moles of Al, and we need 0.02 moles for the reaction, then
(0.2 - 0.02) = 0.18 moles remain after the reaction is complete.
0.18 mol . 26.98g /1mol = 4.86 g of Al remain after the reaction goes complete.
As the limting reactant is the HCl, we work with it to determine the mass of salt which is produced:
6 mol of HCl can produce 2 mol of chloride
Then 0.06 moles of HCl will produce (0.06 . 2) /6 = 0.02 mol of AlCl₃
We convert to mass: 0.02 mol . 133.33g/1mol = 2.67 g of AlCl₃ are made in this reaction.
Let's find out the volume of hydrogen produced, at STP
6 moles of HCl can produce 3 moles of H₂
0.06 moles of HCl will produce (0.06 . 3) /6 = 0.03 moles of H₂
1 mol of any gas at STP occupies 22.4L
0.03 moles of H₂ will ocuppy (22.4 L . 0.03 mol)/1mol = 0.672L
if the same amount of heat is added to 50.0 g samples of each of the metals, which are all at the same temperature, which metal will reach the highest temperature?
Answers
The metal which will reach the highest temperature is the metal with the lowest specific heat capacity.
What is the amount of heat added to each metal?The amount of heat Q = mcΔT where
m = mass of metalc = specific heat capacity of mateal and ΔT = temperature changeTemperature change of the metalMaking ΔT subject of the formula, we have
ΔT = Q/mc
Given that Q and m are the same for each metal,
ΔT ∝ 1/c
We see that the temperature change is inversely proportional to the specific heat capacity.
Since the metals are at the same temperature, the metal which will reach the highest temperature is the metal with the lowest specific heat capacity.
So, the metal which will reach the highest temperature is the metal with the lowest specific heat capacity.
Learn more about temperature here:
https://brainly.com/question/16559442
#SPJ12
If you triple the force on an object , the acceleration will
a
reduced by 1/3
b
double
c
tripled
d
halve
Answers
Answer:
C triple
Explanation:
please crown me
All of the following are examples of primary air pollutants except (a) sulfur dioxide. (b) carbon dioxide. (c) tropospheric ozone. (d) nitrogen oxide. (e) particulates.
Answers
particulates Is not an examples of primary air pollutants , but sulfur dioxide. carbon dioxide. tropospheric ozone. nitrogen oxide is a example of air pollutant.
What exactly is ozone?Three gasses (O3) combine to form the odorless, colorless gas known as ozone, which occurs naturally in the atmosphere. Both the upper atmosphere of the Earth, known as the stratosphere, and the lower atmosphere, known as the troposphere, can contain it.
What are the ozone's sources?Ozone is created in the atmosphere as a result of chemical interactions involving pollutants released from various sources, including as paint evaporation, combustion, consumer products, factories, and other industrial sources.
To know more about air pollutants visits:
https://brainly.com/question/21683809
#SPJ1
A gas sample occupies 2.1 L at a pressure of 101 kPa.
What volume will it occupy if the pressure is increased to 235 kPa?
A. 4.9 L
B. 1.6L
C. 1.2 L
D. 0.9 L
Answers
Answer:
D) 0.9 L
Explanation:
At constant temperature,
PV = Constant
so,
P1.V1 = P2. V2
101 × 2.1 = 235 × V2
V2 = 0.9 L
Answer:
0.9L
Explanation:
Given parameters:
Initial volume = 2.1L
Initial pressure = 101kPa
Final pressure = 235kPa
Unknown:
Final volume = ?
Solution:
The relationship is between pressure and volume. To solve this problem, we apply Boyle's law which states that "the volume of a fixed mass of a gas varies inversely as the pressure changes if the temperature is constant".
Mathematically;
P₁V₁ = P₂V₂
P₁ is the initial pressure
V₁ is the initial volume
P₂ is the final pressure
V₂ is the final volume
Now insert the parameters and solve;
2.1 x 100 = 235 x V₂
V₂ = 0.89L
Use the balanced equation to solve the problem.N2 + 3H22NH323.0g NH3 are made.How many liters of H₂ gas reacted at Stp? L
Answers
By using the ideal gas law to get volume we have"
\(V=\frac{nRT}{P}\)Where v is volume, T is temperatute, n is number of moles, R is the molar gas constant and P is pressure. At STP P= 101,325 Pa, T= 273.15 K and R= 8.314 J/mol K
\(\begin{gathered} \frac{RT}{P}=0.022414cm^3mol^{-1} \\ \\ V=0.0022414n \end{gathered}\)We must first convert mass to moles:
\(\begin{gathered} mole=\frac{mass}{molecular\text{ }mass} \\ mole=\frac{23.0g}{17.0g\text{ }mol^{-1}} \\ \\ mole=1.35 \end{gathered}\)\(To\text{ }determine\text{ }the\text{ }moles\text{ }of\text{ }H2\text{ }gas\text{ }reacted\text{ }we:\frac{2}{3}\times1.35=0.87\text{ }mol\)By substituting this value into the ideal gas law we have:
\(\begin{gathered} V=0.0022414cm^3mol^{-1}\times0.87mol \\ V=0.0019502cm^3 \\ \\ V=1.9502\times10^{-6}L \end{gathered}\)1.9502e-6L of H2 gas reacted at STP
Which statement best describes two functions of blood?
Answers
Answer:
flows through your viens and moves to your blood vesils
Explanation:
its how blood operates
Un móvil avanza con mru a razón de 25 m/s durante 2,54 h. Cuál es la distancia recorrida por el móvil
Answers
Answer:
La distancia recorrida por el móvil es 228.600 m
Explanation:
El movimiento rectilíneo uniforme MRU es el movimiento que describe un cuerpo o partícula a través de una línea recta a velocidad constante. Es decir:, en este caso el movimiento es lineal en una única dirección y la velocidad de desplazamiento es constante.
La velocidad es una magnitud física que expresa la relación entre el espacio recorrido por un objeto y el tiempo empleado para ello mediante la expresión:
\(velocidad=\frac{distancia}{tiempo}\)
En este caso:
velocidad= 25 m/sdistancia= ?tiempo= 2,54 h= 9144 s (siendo 1 h= 3600 s)Reemplazando:
\(25 \frac{m}{s} =\frac{distancia}{9144 s}\)
y resolviendo obtienes:
distancia= 25\(\frac{m}{s}\) *9144 s
distancia= 228.600 m
La distancia recorrida por el móvil es 228.600 m
A movement is rectilinear uniform when an "object" (for example) travels in a straight path at a constant speed, given that its acceleration is zero.
To start solving an exercise, we obtain the data:Velocity (v) = 25m/s
Distance (d) = ?
Time (t) = 2.54hr = 9,144 s
Looking at the data, we see that the time is in hr, but we need it in seconds. Therefore we do a conversion from hours to minutes, taking into account that 1 hour is equal to 3600 seconds.
2,54 hr * (3600 sec / 1 h ) = 9144 secTo calculate the distance traveled by the mobile, the speed is multiplied by the time taken.
For this the following formula is applied:
d = v * tWe apply the data in the formula; to solve for the distance.
d = 25 m/s * 9144 sd = 228, 600 mAnswer: The distance traveled by the mobile is 228,600 meters.