Answers
The heat of combustion per gram of quinone is -25.43 KJ/g.
A constant-volume bomb calorimeter can be used to calculate a substance's heat of combustion. The heat absorbed by the bomb calorimeter can be compared to the heat emitted by the reaction. Additionally, it is necessary to understand the calorimeter's heat capacity.- Q(rxn) = Q(cal) = C(cal)ΔTGiven,
mass of sample = 2.300g
total heat capacity = 7.854 kJ/∘C
Temperature change = 32.09 - 24.64 = 7.45
we have to find out heat of combustion per gram of quinone.
- Q(rxn) = Q(cal) = C(cal)ΔT
Q(rxn) = -(7.854kJ/⁰C)(32.09 - 24.64)
= -58.5 KJ
Now, we will calculate heat of combustion per gram.
Q(rxn) = -58.5/2.3g = -25.43 KJ/g
negative sign denote exothermic reaction.
Thus, heat of combustion per gram of quinone is -25.43 KJ/g.
Learn more about heat of combustion here:
https://brainly.com/question/25312146
#SPJ9
Related Questions
For the compounds H2Te, H2Se, H2S, H2O the general trend is that boiling point increases as the _______ increases. This trend is observed because electrons are held more _____ in H2Te when compared to the other molecules resulting in a stronger induced ________.
Answers
For the compounds H2Te, H2Se, H2S, H2O the general trend is that boiling point increases as the molecular weight increases. This trend is observed because electrons are held more tightly in H2Te when compared to the other molecules resulting in a stronger induced dipole interaction.
The boiling point of a substance is related to its molecular weight, as well as its molecular structure. In the case of H2Te, H2Se, H2S, and H2O, the boiling point increases as the molecular weight increases.
This trend is because the electrons in H2Te are held more tightly compared to the other molecules, leading to a stronger induced dipole-dipole interaction between the molecules. This stronger interaction requires more energy to overcome, resulting in a higher boiling point.
Learn more about boiling point:
https://brainly.com/question/28203474
#SPJ4
Jen and her partner were assigned the Zn/Zn cathode/anode pair which they used to construct their electrolytic cell. They decided to keep the concentration of the corresponding Zn2+ solution constant at 1 M. If Jen ran her cell for 22.8 mins, what current (mA) did she use in order to transfer 9 x 10^-4 moles of electrons?
Answers
Answer:
63.2 mA
Explanation:
We all know that:
Current\(I = \dfrac{Q}{t}\)
which can be further expressed as:
\(I = \dfrac{ne}{t}\)
where;
n = number of electrons
e = charge on electrons
t = time (second)
Then; by replacing the values in the question, we have:
\(\dfrac{(9*10^{-4})\times6\times10^{23}\times1.6\times10^{-19}}{22.8\times60}\)
\(= 0.06316 A \\ \\ \mathbf{I= 63.2 \ mA}\)
Which of the following is an example of something that is made of cells?
A flower
A ball
A sock
A television
Answers
1 mole of sulfur atoms has how much mass
Answers
Answer:
One atom of sulfur has a mass of 32.07 AMU; one mole of S atoms has a mass of 32.07 g.
Explanation:
Therefore, the answer should be 32.07 g
Describe the advantages of the hydrogen-rich fuel cell when compared to the conventional electrochemical cells such as lead-acid battery. (4)
Answers
The hydrogen-rich fuel cell offers advantages in terms of efficiency, environmental impact, operating time, refueling speed, weight, size, and lifespan when compared to conventional electrochemical cells like the lead-acid battery.
The hydrogen-rich fuel cell offers several advantages over conventional electrochemical cells like the lead-acid battery. Here are some of the key advantages:
1. Higher Efficiency: Hydrogen fuel cells have higher energy conversion efficiencies compared to lead-acid batteries. Fuel cells can convert chemical energy directly into electrical energy with minimal loss, while lead-acid batteries have inherent energy losses due to factors such as internal resistance and heat dissipation.
2. Clean and Environmentally Friendly: Hydrogen fuel cells produce electricity through the reaction of hydrogen and oxygen, with water being the only byproduct. They do not produce harmful emissions or contribute to air pollution, making them a cleaner and more sustainable power source compared to lead-acid batteries, which require the use of chemicals like sulfuric acid.
3. Longer Operating Time: Fuel cells have longer operating times compared to lead-acid batteries. Lead-acid batteries have a limited capacity and need to be recharged frequently, while fuel cells can continuously generate electricity as long as there is a supply of hydrogen.
4. Faster Refueling: Refueling a fuel cell is faster compared to recharging a lead-acid battery. Fuel cells can be refueled by replenishing the hydrogen supply, which can be done relatively quickly. In contrast, lead-acid batteries require a longer time to recharge, typically hours, depending on the battery's capacity and charging rate.
5. Lighter Weight and Compact Size: Hydrogen fuel cells have a higher energy density compared to lead-acid batteries, meaning they can store more energy in a smaller and lighter package. This makes fuel cells more suitable for applications where weight and space are critical, such as in portable devices or electric vehicles.
6. Longer Lifespan: Fuel cells generally have a longer lifespan compared to lead-acid batteries. Lead-acid batteries can experience degradation over time due to factors like sulfation, which can reduce their overall capacity and lifespan. Fuel cells, on the other hand, can provide consistent performance over an extended period with proper maintenance.
These advantages make fuel cells a promising technology for various applications, including transportation, stationary power generation, and portable electronics.
for more questions on fuel cell
https://brainly.com/question/14122421
#SPJ8
When steam comes out of the tea kettle, what TWO types of energy are visible?
mechanical
chemical
electrical
thermal
light
Answers
thermal steam comes out of the tea kettle, what TWO types of energy are visible
What kind of energy does steam emit as it heats up?Latent heat has potential energy and sensible heat has kinetic energy in the case of steam. As water is heated, an invisible gas called steam is produced. It is liquid water that has been converted to gas. Saturated steam is steam that has come into direct touch with the water it is being produced from.
The majority of the energy contained in the hot, gaseous steam is released as it expands and cools as it passes by the rotating blades of the turbine. The blades are constantly being spun by this steam. Hence, the blades mostly transform the potential energy of the steam into kinetic energy.
learn more about thermal steam
https://brainly.com/question/18261311
#SPJ1
How many kilograms (kg)are there in 2.650 tons?[?] kgMass in kgEnter
![How many kilograms (kg)are there in 2.650 tons?[?] kgMass in kgEnter](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/k07PgrSCC0rX47N9MV2ZNA9C3pV2A3T5.jpeg)
Answers
ANSWER
Mass in kg = 2,403.9475 kg
STEP-BY-STEP EXPLANATION:
Given information
From the question provided, you are asked to convert 2.650 tons to kilograms
Let x be the mass converted in kg
Recall that, 1 ton is equivalent to 907.15kg according to the Standard International unit
\(\begin{gathered} 1\text{ ton }\rightarrow\text{ 907.15kg} \\ 2.650\text{ tons }\rightarrow\text{ xkg} \\ \text{Cross multiply} \\ 1\cdot\text{ x = 2.650 }\cdot\text{ 907.15} \\ x\text{ = 2,403.9475 kg} \end{gathered}\)Therefore, we have 2,403.9475kg in 2.650 tons
Given the law of conservation of energy, what happens when a 200°C iron bar is placed in thermal contact with a 30°C block of wood?
Answers
When a 200°C iron bar is placed in thermal contact with a 30°C block of wood, energy leaves the iron bar and enters the wood until the temperatures are equal.
Law of conservation of energy states that the energy cannot be lost or formed but it can only be transformed from one form to another.
According to the given question, the block of wood is at a lower temperature than an iron bar. Hence, heat will flow from the iron bar to the block of wood until the temperatures of both are equal.
Know more about the Law of conservation of energy,
https://brainly.com/question/24772394
https://brainly.com/question/11549071
A 25 ml sample of 1.2 molar potassium chloride mix with 15 ml of 0.90 molar barium nitrate solution and precipitate reaction occurs twice case LX + BA no3s aqueous bacl2 solid + 2ks what is the practical yield percentage yield mass is 2.45 g
Answers
theoretical yield) × 100%
Percentage yield = (2.45 g / 2.81 g) x
100%
Percentage yield = 87.2%
Therefore, the practical yield percentage yield is 87.2%, and the mass of the BaCI2 produced is 2.81 g.
Moles of KCI = volume (in L) X
concentration
Moles of KCI = (25/1000) L x 1.2 mol/L
= 0.03 mol
Moles of Ba(NO32 = volume (in L) X
concentration
Moles of Ba(NO3)2 = (15/1000) L x
0.90 mol/L = 0.0135 mol
Moles of BaCI2 formed = 0.0135 mol
The molar mass of BaCI2 is 208.23 g/ mol, so the mass of BaCI2 produced is:
Mass of BaCI2 = moles of BaC12 x
molar mass of BaCI2
Mass of BaCI2 = 0.0135 mol x 208.23
g/mol
Mass of BaCI2 = 2.81 g
which 2 organ systems work together to break down and deliver food molecules to cells
A. Cardiovascular and immune
B. Nervous and muscular
C. Digestive and cardiovascular
D. Respiratory and cardiovascular
PLEASE HURRY I NEED THIS ANSWER
Answers
Answer:
C
Explanation:
The stomach digests the food (Digestive System) are the nerves and they send the protein etc.
An atom has 24 protons, 22 electrons, and 28 neutrons. What is the IONIC CHARGE of the atom? *
Answers
Answer:
-4
Explanation:
An atom becomes charged when the number of protons does not equal the number of electrons. For example, if an element has six protons but only five electrons, the net charge of the element is +1. Conversely, if an element has six protons but seven electrons, then the net charge of the element is -1.
matching will give brainliest. if you can answer any it help
1. element with atomic number greater than 92.
2. helium nucleus with and atomic number or 2 and a mass of 4.
3. unit for measuring exposure to radiation.
4. changing of one element to another due to alpha or beta decay.
5. caused by the decay of an electron.
6. atoms of the same element with different numbers of neutron.
7. the initial isotope before decay.
a. rem
b. alpha particle
c. beta particle
d. trans uranium element
e. transmutation
f. isotope
g. parent nuclide
Answers
Explanation:
In natural radioactive decay, three common emissions occur. When these emissions were originally observed, scientists were unable to identify them as some already known particles and so named them:
alpha particles ( α )
beta particles (β)
gamma rays (γ)
These particles were named using the first three letters of the Greek alphabet. Some later time, alpha particles were identified as helium-4 nuclei, beta particles were identified as electrons, and gamma rays as a form of electromagnetic radiation like x-rays, except much higher in energy and even more dangerous to living systems.
The specific heat capacity of liquid water is 4.18 J/g oC. Calculate the quantity of energy required to heat 1.50 g of water from 26.5oC to 83.7oC. (Ignore significant figures for this problem.)
Answers
Answer: The quantity of heat required is 358.644 J.
Explanation:
Given: Specific heat capacity = \(4.18 J/g^{o}C\)
Mass = 1.50 g
\(T_{1} = 26.5^{o}C\)
\(T_{2} = 83.7^{o}C\)
Formula used to calculate heat energy is as follows.
\(q = m \times C \times (T_{2} - T_{1})\)
where,
q = heat energy
m = mass
C = specific heat capacity
\(T_{1}\) = initial temperature
\(T_{2}\) = final temperature
Substitute the values into above formula as follows.
\(q = m \times C \times (T_{2} - T_{1})\\= 1.50 \times 4.18 J/g^{o}C \times (83.7 - 26.5)^{o}C\\= 358.644 J\)
Thus, we can conclude that quantity of heat required is 358.644 J.
How many moles of aluminum oxide are produced according to the reaction below given that you start with 40.0 grams of Al and 19.0 grams of O2?
Reaction: 4Al + 3O2 --> 2Al2O3
A. 0.185
B. 0.741
C. 5.00
D. 0.396
Answers
From the stoichiometry of the reaction, 0.74 moles of aluminium oxide is produced.
The equation of the reaction is;
4Al + 3O2 --> 2Al2O3
Number of moles of Al = 40.0/27 g/mol = 1.48 moles
Number of moles of O2 = 19.0 g/32 g/mol = 0.59 moles
Now;
4 mols of Al reacts with 3 moles of O2
1.48 moles of Al reacts with 1.48 moles × 3 moles/4 mols
= 1.11 moles
We can see that O2 is the reactant in excess.
Hence;
4 moles of Al produces 2 moles of aluminium oxide
1.48 moles of Al produces 1.48 moles × 2 moles/4 moles = 0.74 moles of aluminium oxide
Learn more about stoichiometry: https://brainly.com/question/9743981
Which factors play a role in creating ocean waves
Answers
Answer: wind speed, direction, duration, and fetch
Explanation: Ocean waves are usually produced by the wind that moves its energy to the water
You have 4 litres of a 3.0 mol/L solution of NaCl in a chemical store room.
How many moles of NaCl are present?
Answers
Answer:
12
Explanation:
nNaCl= 4x3=12
A chemical compound has a molecular weight of 89.05 g/mole. 1.400 grams of this compound underwent complete combustion under constant pressure conditions in a special calorimeter. This calorimeter had a heat capacity of 2980 J °C.1 (Note that the calorimeter was made of a metal shell, a water "substitute" - a special oil, and a thermocouple). The temperature went up by 11.95 degrees.
Required:
Calculate the molar heat of combustion of the compound.
Answers
Answer:
\(\Delta _{comb}H=-2,265\frac{kJ}{mol}\)
Explanation:
Hello!
In this case, for such calorimetry problem, we can notice that the combustion of the compound releases the heat which causes the increase of the temperature by 11.95 °C, it means that we can write:
\(Q _{comb}=-C_{calorimeter}\Delta T_{calorimeter}\)
In such a way, we can compute the total released heat due to the combustion considering the calorimeter specific heat and the temperature raise:
\(Q _{comb}=-2980\frac{J}{\°C} *11.95\°C\\\\Q _{comb}=-35,611J\)
Next, we compute the molar heat of combustion of the compound by dividing by the moles, considering 1.400 g were combusted:
\(n=1.400g*\frac{1mol}{89.05g} =0.01572mol\)
Thus, we obtain:
\(\Delta _{comb}H=\frac{Q_{comb}}{n}=\frac{-35,611J}{0.01572mol} \\\\\Delta _{comb}H=-2,265,331\frac{J}{mol}*\frac{1kJ}{1000J} \\\\\Delta _{comb}H=-2,265\frac{kJ}{mol}\)
Best regards!
Write the complete symbol, including mass number and atomic number, for each atom.
contains 28 protons and 30 neutrons:
contains 22 protons and 21 neutrons:
contains 15 electrons and 19 neutrons
an oxygen atom with 10 neutrons:
Answers
The complete symbol, including mass number and atomic number, for each atom serially is given as, ²⁸₃₀Ni,²²₂₁Ti,¹⁵₁₉P,O-18 .
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ9
deprotonate enolate enone
what do these terms mean?
Answers
Deprotonation refers to the removal of a proton from a molecule, while an enolate is an anionic species formed by deprotonation of the α-carbon adjacent to a carbonyl group. An enone, on the other hand, is a molecule containing a carbon-carbon double bond and a carbonyl group.
"Deprotonate," "enolate," and "enone" are terms used in organic chemistry to describe specific reactions and functional groups. Let's break down each term:
Deprotonate: Deprotonation refers to the removal of a proton (H+) from a molecule. It is a process that involves the transfer of a proton from a molecule to a base. The resulting species is negatively charged and called an anion.
Deprotonation reactions are common in various organic reactions and play a crucial role in the formation of new bonds and the generation of reactive intermediates.
Enolate: An enolate is an anionic species that contains a carbon-carbon double bond and a negatively charged oxygen or nitrogen atom. Enolates are formed through deprotonation of the α-carbon adjacent to a carbonyl group (such as a ketone or aldehyde).
The formation of enolates is an important step in many organic reactions, such as aldol condensation and Michael addition, as enolates serve as nucleophiles or reactive intermediates.
Enone: An enone is a molecule that contains a carbon-carbon double bond (C=C) and a carbonyl group (C=O) adjacent to each other. Enones are carbonyl compounds that possess a conjugated double bond system. They exhibit unique reactivity due to the presence of both a double bond and a carbonyl group, making them valuable intermediates in organic synthesis.
Enones are involved in various reactions, including Michael additions, Diels-Alder reactions, and cycloadditions, to form complex organic compounds.
For more such question on Deprotonation. visit :
https://brainly.com/question/28480664
#SPJ8
Question 4
1 pts
How many moles of chromium are 1.5 x 10^24 atoms?
Answers
Answer:
What event prompted the persian Gulf War?
Answer:
Using N = n × L
L = Avogadro's constant =6.02 ×10^23
n = number of moles
N = Number of entities
N = 1.5 × 10^24
n = N /L
n = 1.5×10^24/6.02×10^23
n = 2.49 moles
Hope this helps.
The scientific theory of plate tectonics states that the Earth's crust is composed of many plates, or slabs of rock. Interactions of these plates have created different landforms, or geologic features. Which of the following statements provides evidence for this theory?
A.
A large earthquake off the coast of Japan induces a wave that is over twenty feet high.
B.
An iceberg buckles the hull of the Titanic and allows water to enter between the steel plates.
C.
The city of Pompeii is perfectly preserved under volcanic ash following the eruption of Mount Vesuvius.
D.
Fossilized marine creatures can be found at the top of the Himalayan Mountains.
Answers
Answer:
study island
proof
Explanation:

Fossilized marine creatures found at the top of the Himalayan Mountains provide evidence for a scientific theory of plate tectonics. Therefore, option (D) is correct.
What is Plate tectonics?Plate tectonics can be explained as the Earth's lithosphere being made of a large number of tectonic plates that have been slowly moving since about 3.4 billion years ago.
The lithosphere is broken into 7 or 8 major plates and many minor plates or "platelets". These plates meet, and their relative motion evaluates the kind of plate boundary: convergent, transform, or divergent.
Mountain-building, Earthquakes, volcanic activity, and oceanic trench formation take place along these plate boundaries. The relative annual movement of the plates ranges from 0 to 10 cm.
Tectonic plates are comprised of the oceanic and the thicker continental lithosphere. The process of subduction which is actually one plate moving under another carries the edge of the lower plate down into the mantle along convergent boundaries.
Pebbles, Shells, and marine fossils found in the limestone beds of the tallest Himalayas Mountains give evidence of the theory of plate tectonics.
Learn more about plate tectonics, here:
brainly.com/question/19317822
#SPJ6
how can the ph of antiacid table de determine in laboratory
Answers
Answer:
Antacids are bases that react stoichiometrically with acid. The number of moles of acid that can be neutralized by a single tablet of a commercial antacid will be determined by back titration.
Correct me if i'm wrong, Hope This helps
Favor(Brainliest)
#Carryonlearning
Given the isotopic symbol below- how many neutrons are present in this
beryllium ion? Answer with just a number*
1 point
9
+2
Be
4.

Answers
Answer:
5 neutrons.
Explanation:
The following data were obtained from the question:
Mass number = 9
Atomic number = 4
Charge = +2
Neutron number =.?
Next, we shall determine the number of protons in the beryllium ion. This can be obtained as follow:
The atomic number is simply the number protons present in the atom. Thus,
Proton = Atomic number
Atomic number = 4
Proton = Atomic number = 4
Finally, we shall determine the Neutron number as follow:
Mass number = 9
Proton number = 4
Neutron number =.?
Mass number = Proton + Neutron
9 = 4 + Neutron
Collect like terms
Neutron = 9 – 4
Neutron = 5
Thus, there are 5 neutrons b present in the beryllium ion.
What kind of substances can you not heat on a Bunsen burner?
Answers
Answer:
Substances which are non heat-resistant and volatile organic liquid with flammable vapor.
Explanation:
Bunsen Burner are used as a source of fire in the laboratory for heating up substances. Extra care however should be taken during heating in order to prevent fire or other forms of accidents.
Heating of mind heat resistant substances should be frowned at as the substances may get heated up and melt thereby exposing the liquid substances which may be flammable to fire thereby causing fire outbreak.
Heating of volatile organic liquid with flammable vapor should also be discouraged to prevent fire accidents.
How much water has to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M?
Answers
Approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
To find the amount of water that needs to be evaporatedThe relationship between the initial and final concentrations and volumes must be taken into account.
Given: Initial concentration \((C^1) = 1 M Initial volume (V^1) = 250 mL\)
\((C^2) = 3 M final concentration\)
We can use the equation:
\(C^1 * V^1 = C^2 * V^2\)
Where:
\(V^2\)is the final volume of the solution
Rearranging the equation to solve for V2:
\(V^2 = (C^1 * V^1) / C^2\)
Substituting the given values:
\(V^2 = (1 M * 250 mL) / 3 M\)
\(V^2 = 250 mL / 3\)
\(V^2\) ≈ \(83.33 mL\)
To find the amount of water that needs to be evaporated, we subtract the final volume from the initial volume:
Amount of water to be evaporated = \(V^1 - V^2\)
Amount of water to be evaporated = 250 mL - 83.33 mL
Amount of water to be evaporated ≈ 166.67 mL
Therefore, approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
Learn more about Initial concentration here: brainly.com/question/30720317
#SPJ1
Which is least likely to occur after an experiment i conducted to test a hypothesis?
O The data is analyzed to see if it supports or refutes the hypothesis.
O The same experiment is conducted again to see if the data are reliable.
O The hypothesis becomes a theory if the results support it.
O Anew experiment is designed to provide additional data about the hypothesis.
Answers
Answer:
The hypothesis becomes a theory if the results support it is answer.
Explanation:
I hope it's helpful!
balanced equation for beta decay of iodine-138
Answers
Answer:
Explanation:
Please take a look at the attached picture about the general formula of beta decay and the answer.
beta decay is an atom breaking down into a new atom of another element which the atomic number is 1 larger than the original, and one electron (β particle). The mass no. of the new atom remains same as before.
138 is iodine's mass number, the atomic number of iodine is 53, referred from a periodic table. The new atom formed will be Xe, which has an atomic no. of 54.
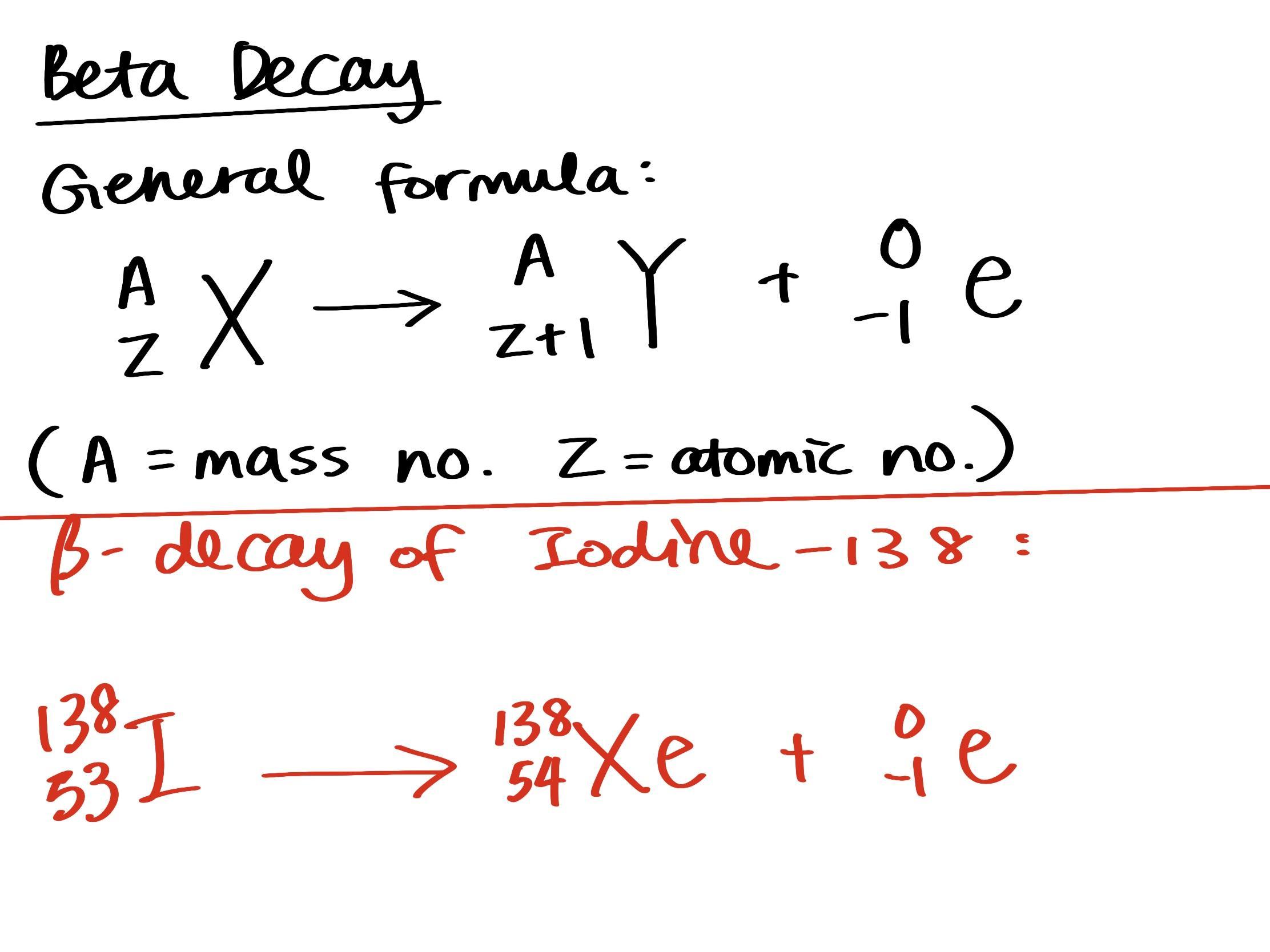
18. Which equation represents an equilibrium system? 2Mg(s) + O₂(g) — 2MgO(s) O = CO₂ (s) CO₂(g) Agt (aq) + Cr (aq) — O 250₂(g) + O₂(g) — AgCl (s) 250, (g)
Answers
An equilibrium reaction occurs when the rates of forward and backward reactions are equal. Therefore, the correct equation representing an equilibrium system is:
2Mg(s) + O₂(g) ⇌ 2MgO(s)
An equilibrium reaction is a reversible reaction in which the rate of the forward reaction is equal to the rate of the backward reaction. It is a state of balance in which the concentrations of reactants and products remain constant over time.An equilibrium equation is a chemical reaction in which the forward and backward reactions are equal and there is no net change in the concentration of reactants or products. A reversible arrow (↔) indicates that the reaction is at equilibrium. The forward and reverse reactions occur at the same rate in an equilibrium reaction.For such more questions on equilibrium reaction
https://brainly.com/question/31383509
#SPJ8
40 grams of Object A reacts with 9.3 grams of Object B to produce
Object AB. What is the mass of object AB?
O 34.70 grams
O 16.10 grams
236.22 grams
O2.73 grams
Answers
According to law of conservation of mass,when object A and B produce object AB the mass of object AB is 49.3 g.
What is law of conservation of mass?According to law of conservation of mass, it is evident that mass is neither created nor destroyed rather it is restored at the end of a chemical reaction .
Law of conservation of mass and energy are related as mass and energy are directly proportional which is indicated by the equation E=mc².Concept of conservation of mass is widely used in field of chemistry, fluid dynamics.
Law needs to be modified in accordance with laws of quantum mechanics under the principle of mass and energy equivalence.This law was proposed by Antoine Lavoisier in the year 1789.
As object A and B combine to give object AB , therefore mass of object AB is 49.3 g.
Learn more about law of conservation of mass,here:
https://brainly.com/question/28711001
#SPJ1
Your question is incomplete, but most probably your full question was,40 grams of Object A reacts with 9.3 grams of Object B to produce
Object AB. What is the mass of object AB?
34.70 grams
16.10 grams
236.22 grams
2.73 grams
49.3 grams
What quantity in moles of chlorine gas at 120.0 °C and 33.3 atm would
occupy a vessel of 36.5 L?
Answers
The number of chlorine moles present in the vessel are 37.67 mol.
What are moles?
Moles are the number of substances present in a system which consists of entities like ions, atoms, or molecules as much as atoms in the weight of pure carbon which is 12 grams. It is the SI unit of amount of substance.
One mole of a substance is equal to the Avogadro Number which is equal to 6.022×10²³.
According to Ideal gas law,
PV=nRT where,
P= Pressure
V= Volume
n= number of moles
R= Ideal gas constant
T= temperature (in Kelvin)
n= PV/RT
⇒33.3×36.5/0.0821×393
⇒1215.45/32.2653
⇒37.670
To learn more about mole concept from the given link
https://brainly.com/question/16488605
#SPJ13