a 100.0-g sample of water has an inital temperature of 25.0C the final temperature of the after heating was 27.0 how many joules of heat is released by the reaction
Answers
836 joules of heat were released by the reaction.
To calculate the heat released by the reaction, we can use the formula q = mcΔT, where q is the heat in joules, m is the mass of the water, c is the specific heat capacity of water, and ΔT is the change in temperature. In this case, the mass (m) is 100.0 g, the initial temperature is 25.0°C, and the final temperature is 27.0°C.
Using the given information, we can determine the amount of heat released as follows:
1. Calculate the change in temperature (ΔT): ΔT = final temperature - initial temperature = 27.0°C - 25.0°C = 2.0°C.
2. Use the specific heat capacity of water (c), which is 4.18 J/g°C.
3. Apply the formula q = mcΔT: q = (100.0 g)(4.18 J/g°C)(2.0°C) = 836 J.
Thus, 836 joules of heat were released by the reaction.
Know more about Specific Heat Capacity here:
https://brainly.com/question/29766819
#SPJ11
Related Questions
Sulfur is a non metallic material that has a density of 2g/cm3. The volume of a sample of sulfur was measured to be 5.0cm3. What is the density of the wood?
Answers
question: Sulfur is a non metallic material that has a density of 2g/cm3. The volume of a sample of sulfur was measured to be 5.0cm3. What is the mass of the sulfur
Answer:
0.01 kg
Explanation:
Density: This can be defined as the ratio of mass of a body and it's volume. The S.I unit of volume is kg/m³.
Therefore,
Density = mass/volume
D = m/V......................................................... Equation 1
making m the subject of the equation
m = D×V ................................... Equation 2
Given: D = 2 g/cm³ = 2000 kg/m³, V = 5.0 cm³ = 0.000005 m³
subtitute into equation 2
m = 2000×0.000005
m = 0.01 kg or 10 g
if 140cm3 of methane diffuse into air in 72 sec,how long will it take 210cm3 of sulphur dioxide to diffuse under the same condition ?
Answers
Answer:
time for sulphur dioxide to diffuse is 221.05 secs
The time for sulphur dioxide to diffuse is 108 sec.
Which gas diffuses twice quickly as SO2?Sulfur dioxide has a molecular weight of sixty-four and as a result, the square root is 8. since its inversely proportional, on the way to diffuse two times as fast, the rectangular root of the molecular weight should be 4, and subsequently the molecular weight might be 16. So the solution is methane (CH4).
Which gases diffuse the fastest?The charge of diffusion for a gas is inversely proportional to the rectangular root of its molecular mass (Graham's law). The gasoline with the lowest molecular weight will effuse the fastest. The lightest, and consequently fastest, fuel is helium.
Learn more about Sulfur dioxide here: https://brainly.com/question/15654465
#SPJ2
What does this demonstration show?
Answers
cal is titrating 52.5 ml of 0.304 m hbr with 0.344 m ba(oh)2. how many ml of ba(oh)2 does cal need to add to reach the equivalence point?
Answers
If cal is titrating 52.5 ml of 0.304 m hbr with 0.344 m ba(oh)2. The amount of ml of ba(oh)2 that cal need to add to reach the equivalence point is : 92.79 ml.
What is the amount of ml of ba(oh)2?In order to solve this problem, we can use the following equation that relates the moles of acid to the moles of base:
moles of acid = moles of base
We can rearrange this equation to solve for the volume of base needed to reach the equivalence point:
volume of base = moles of acid / concentration of base
First, let's calculate the number of moles of HBr in the solution:
moles of HBr = volume of HBr x molarity of HBr
moles of HBr = 52.5 ml x 0.304 mol/L
moles of HBr = 15.96 mmol
Since the reaction between HBr and Ba(OH)2 is a 1:2 acid-base reaction, we know that the number of moles of Ba(OH)2 needed to reach the equivalence point is twice the number of moles of HBr:
moles of Ba(OH)2 = 2 x moles of HBr
moles of Ba(OH)2 = 2 x 15.96 mmol
moles of Ba(OH)2 = 31.92 mmol
Finally, we can use the equation above to calculate the volume of Ba(OH)2 needed:
volume of Ba(OH)2 = moles of HBr / concentration of Ba(OH)2
volume of Ba(OH)2 = 31.92 mmol / 0.344 mol/L
volume of Ba(OH)2 = 92.79 ml
Therefore, Cal would need to add 92.79 ml of 0.344 M Ba(OH)2 to reach the equivalence point.
Learn more about amount of ml of ba(oh)2 here:https://brainly.com/question/24206836
#SPJ1
convert 8.7x10^24 atoms of ammonium chloride to grams of ammonium cloride
Answers
The mass of 8.7x10^24 atoms of ammonium chloride would be 772.93 grams.
Mass of atoms of substancesAccording to standards, 6.022 x \(10^{23\) atoms of any substance is equivalent to 1 mole of the substance.
6.022 x \(10^{23\) atoms = 1 mole
8.7 x \(10^{24\) atoms of ammonium chloride = 8.7 x \(10^{24\) /6.022 x \(10^{23\)
= 14.45 mol of ammonium chloride.
The molar mass of ammonium chloride is 53.49 g/mol.
The mass of 14.45 mol ammonium chloride would be:
14.45 x 53.49 = 772.93 grams
In other words, 8.7 x \(10^{24\) atoms of ammonium chloride is equivalent to 772.93 grams of ammonium chloride.
More on moles and atoms of substances can be found here: https://brainly.com/question/28864489
#SPJ1
Which element will gain one electron in an ionic bond?
Select one:
a. Al
b. Cu
c. Br
d. O
Answers
Answer:
C. Bromine
Explanation:In ionic bond , the metals looses electron and the non metal gains electron.
In the given options ;
Aluminum (Al)= METAL
Copper (Cu) = METAL
Bromine (Br) = NON METAL
Oxygen (O) = NON METAL
That leaves us with bromine and oxygen.
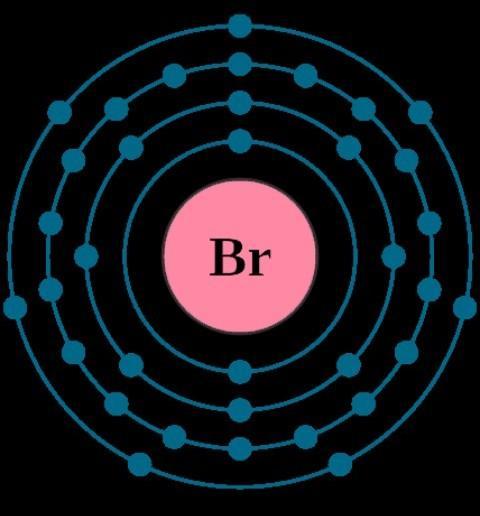
Oxygen has an electronic configuration of 2,6.(see image).
So oxygen needs to gain two electrons.
Bromine has one valence electron ( see image).
So therefore , Bromine needs to gain one electron as It is in Group VII



Li = Li+ + e- oxidation or reduction?
Answers
Neutral lithium is oxidized and changed into Li+ at the anode. When the electrons from the anode reaction are received at the cathode, this causes the reduction of Co(IV) to Co(III).
What is the function of oxidation?The biological aerobic system (BOD) of wastewater is decreased after oxidation, which also lessens some contaminants' cytotoxicity. Some contaminants undergo this treatment and are transformed into dioxide, water, and sludges. Disinfection is commonly accomplished using chemical oxidation.
What causes oxidation?The reaction brought on by coming into touch with oxygen molecules is known as oxidation. These materials can be non-metals like live tissues or metals. Technically speaking, oxidation is the removal of one electrons during the phase of interaction between two or more atoms.
To know more about Oxidation visit:
https://brainly.com/question/16976470
#SPJ13
When 1 mole of N2(g) reacts with O2(g) to form NO2(g) according to the following equation, 66.4 kJ of energy are absorbed.
N2(g) + 2 O2(g) When 1 mole of N2(g) reacts wi 2 NO2(g)
Is this reaction endothermic or exothermic? endothermic exothermic
What is the value of q? kJ ...
Answers
The value of q is 66.4 kJ.
The given chemical equation is shown below.
N₂(g) + 2 O₂(g) = 2NO₂(g) (ΔH) = +66.4 kJ/mol
Since the enthalpy change (ΔH) is positive, it means that heat is absorbed during the reaction, so this type of reaction is known as an endothermic reaction.
The amount of heat absorbed (q) can be calculated as shown below.
q = nΔH
where,
n is the number of moles of reactant.
For this reaction, 1 mole reacts, so n = 1.
The amount of heat absorbed is shown below.
q = (1 mol) × (66.4 kJ/mol) = 66.4 kJ
So, the value of q is 66.4 kJ.
To learn about heat absorbed
https://brainly.com/question/29406646
#SPJ4
How many moles of nahco3 are in 1750 ml of a 0.26 m solution?
Answers
To find the number of moles of NaHCO3 in 1750 ml of a 0.26 M solution, we need to use the formula: moles = concentration x volume
First, we need to convert the volume from milliliters to liters:
1750 ml = 1.75 L
Now we can plug in the values we have:
moles = 0.26 M x 1.75 L
moles = 0.455 moles
Therefore, there are 0.455 moles of NaHCO3 in 1750 ml of a 0.26 M solution. To calculate the number of moles of NaHCO3 in a 0.26 M solution with a volume of 1750 mL.
You can use the formula: moles = molarity × volume (in liters)
First, convert the volume from mL to liters: 1750 mL = 1750/1000 = 1.75 L
Now, plug the values into the formula:
moles of NaHCO3 = 0.26 M × 1.75 L = 0.455 moles
So, there are 0.455 moles of NaHCO3 in the 1750 mL of the 0.26 M solution.
To know more about moles visit :-
https://brainly.com/question/30885025
#SPJ11
Select all the correct answers.
Which two statements are true about energy transformations?
Energy is never released from an object as heat.
Energy is never transformed between kinetic energy and potential energy.
O Energy is never created.
Energy is never completely transformed.
Energy is never destroyed.
Reset
Next
Answers
True Statements
Energy is never destroyedEnergy is never completely transformedEnergy is never createdFalse Statements
Energy is never released from an object as heatEnergy is never transformed between kinetic energy and potential energyIs CO2 polar or nonpolar electronegativity?
Answers
Take the carbon dioxide (CO2) molecule as an illustration. The linear CO2 molecule has polar carbon-oxygen double bonds (electronegativities: C = 2.5, O = 3.5).
Is CO2 electronegativity polar?
The polar bonds in CO2 and H2O are both two. The CO2 molecule is non-polar because the dipoles in the linear form of the molecule cancel each other out.
Is the bond in CO2 polar or nonpolar?
While sulphur dioxide is a bent molecule, carbon dioxide is a linear molecule. In spite of the fact that both molecules have polar bonds (see bond dipoles on the Lewis structures below), carbon dioxide is nonpolar whereas sulphur dioxide is polar.
To know more about oxygen visit:
https://brainly.com/question/14962130
#SPJ4
What is dinitrogen heptoxide formula?
Answers
The formula of the compound dinitrogen heptoxide is N₂O₅.
One of the binary nitrogen oxides is the substance Dinitrogen heptoxide.
A compound creates a new product by combining one or more additional ingredients. The mass ratio required to produce that product is equivalent to the combination of two substances or components. It is tightly connected, and they are equivalent in every manner to create a combination.
Dinitrogen heptoxide has the chemical formula N₂O₅.
This molecule has two nitrogen atoms and five oxygen atoms, as can be seen, and its name, Dinitrogen heptoxide, must reflect this
To know more about dinitrogen oxides, visit,
https://brainly.com/question/21479339
#SPJ4
What characteristics of a molecule determine whether or not it is polar?.
Answers
Answer:
The molecules having positively charged end and negatively charged end due to the difference in the charges of atoms present in the molecules are polar molecules. The molecules that do not have such separation of electric charges are nonpolar. Most of the polar molecules have an asymmetric or uneven distribution of electrons.
Explanation:
hope this helps
have an awesome day -TJ
may I please have brainliest
Answer:
The polarity of the individual bonds in the molecule
The arrangement of bonds around the molecule
Explanation:
Hope this helps
What is the mass of 35.0L of oxygen?
Answers
Answer:15.9994 g/mol
Explanation:
How to reduce the volume of hydrogen gas
Answers
At constant temperatures, the simplest approach to reduce the volume of a gas is to raise its pressure. So, at 700 bar, or 700 times normal atmospheric pressure, hydrogen has a density of 42 kg/m3, compared to 0.090 kg/m3 at normal pressure and temperature.
What can hydrogen gas eliminate?Hydrogen decreases metal oxides in the reactivity series below. That is, hydrogen can only decrease the oxides of metals that are less reactive than hydrogen itself.
High-Temperature Water Splitting: Chemical processes that split water to make hydrogen are fueled by high temperatures generated by solar concentrators or nuclear reactors.
The oxidation number of hydrogen gas is 0, but the oxidation state of hydrogen atoms in water is +1. As a result, the hydrogen atom has been oxidized. It acts as a reducing agent.
learn more about hydrogen gas refer
https://brainly.com/question/19813237
#SPJ1
Which prediction best shows what the population could look like after many generations? What caused it to change?
Answers
Prediction 1 best shows what the population could look like after many generations. Because, the long beak traits is having more life time.
What is genetic traits ?Each characteristics in a living thing is created by a respective genetic coding in its body. The genetic code which is responsible for a particular behavior or appearance is called a genetic trait.
Here, the prediction 1 is best . It already saying that long-beak hummingbirds are more likely to survive, that baby survived long enough to pass on its mutation, so the long-beak trait became more common over generations.
Prediction 2 says abut a genetic trait which is less fit to survive. Hence, cannot affect the next generation population. Therefore, prediction 1 is best.
Find more on genetic traits:
https://brainly.com/question/29679846
#SPJ1
Your question is incomplete. But your complete question probably was:
Prediction 1 :Two hummingbirds with short or medium beaks had a baby with a mutation in its genes for the long-beak trait.
Prediction 2 is best. A hummingbird could have been born with a mutation in its genes for the long-beak trait and lived for a little while.
Which of the following would be used to measure the distance from London to Paris? square meter feet meter kilometer
Answers
Answer:
meters
Explanation:
What is the proper value for B in the above table?
A. 65
B. 35
C. 30
D. 63

Answers
Answer:
I dont know it might be A I would wait for someone else to answer
I’m pretty sure
What pairs of aqueous solutions form percitipate when mixed?
Answers
When silver nitrate and sodium chloride are combined with water, silver chloride will solidify and precipitate out of solution. In this instance, silver chloride is the precipitate.
Which four liquid precipitation examples are there?Precipitation includes the following: rain, hail, sleet, and snow. Rain forms when water vapour in clouds condenses on dust particles, which eventually grow too big to stay in the cloud and fall to the ground, where they collect more water and enlarge further.
What does the precipitation reaction in aqueous solution look like as an example?The chemical reaction between potassium chloride and silver nitrate, in which solid silver chloride precipitated out, is among the greatest examples of precipitation reactions. This precipitation reaction resulted in the formation of an insoluble salt.
To know more about precipitate visit:-
https://brainly.com/question/11387485
#SPJ9
Could someone answer this for me?

Answers
Answer:
B
Explanation:
leak testing a low-pressure refrigeration system with nitrogen in excess of 10 psig could cause which of the following to fail
Answers
Leak testing a low-pressure refrigeration system with nitrogen in excess of 10 psig could cause: The system's pressure relief valves.
If a low-pressure refrigeration system is leak tested with nitrogen at a pressure exceeding 10 psig (pounds per square inch gauge), it could cause the system's pressure relief valves to fail.
Pressure relief valves are safety devices installed in refrigeration systems to protect against excessive pressure buildup. They are designed to open and release excess pressure when the system reaches a certain threshold to prevent damage or failure of the system.
When leak testing with nitrogen, it is crucial to ensure that the pressure does not exceed the recommended limits specified by the manufacturer or industry standards.
Exceeding the pressure limits can lead to the overloading of pressure relief valves, causing them to open prematurely or malfunction. This can compromise the safety of the system and potentially result in equipment damage or even personal injury.
It is important to follow proper procedures and guidelines for leak testing refrigeration systems to maintain their integrity and safety.
This includes adhering to recommended pressure limits and utilizing appropriate equipment and techniques to detect and repair any leaks.
To know more about "Refrigeration system" refer here:
https://brainly.com/question/12950632#
#SPJ11
I NEED HELP QUICK
Which of the following has the smallest atomic radius?
F1-
F
Mg
Mg2+
Answers
1. If you combine Mg with Ni2NO3, a reaction occurs. Predict the products from this reaction.
Answers
After adding the sulfuric acid and letting that reaction proceed to completion, what chemical species were left in the beaker?.
Answers
After adding the sulfuric acid and letting that reaction proceed to completion, Copper sulfate and water chemical species were left in the beaker.
Sulfuric acid, also known as sulphuric acid or oil of vitriol in the past, is a mineral acid made up of the elements hydrogen, oxygen, and sulfur with the chemical formula H2SO4. It is a viscous liquid that is miscible with water. It has no color or smell.
When sulfur and copper are combined, copper sulfate, an inorganic substance, is produced. It has the ability to eliminate snails, algae, fungi, plants, roots, and plants. The amount of copper in copper sulfate determines how dangerous it is. Since 1956, it has been legal in the US to use copper sulfate in pesticide products.
To know more about copper sulfate refer to: https://brainly.com/question/21478349
#SPJ4
What is a variable? A. Something that must be kept constant in an experiment. B. A value or characteristic that can take different values. C. A statement that explains a phenomena and can be tested. D. An educated guess used in an experiment.
Answers
Answer:
The answer is B, a value or characteristic that can take different values
Explanation:
ex.) 1s + 3
s is the variable
Variables are the varying or unchangeable data or factors in an experimental design. It can be defined as a characteristic that can have different values. Thus, option B is correct.
What is the meaning of variables?Variables are experimental characteristics that may be consistent or can be changeable. In an experimental design, the variables are used to analyze the cause and effect.
The variables of an experiment can be dependent, independent, or controlled. The varying factors in an experiment to analyze are called independent variables.
The factors that depend and respond to the effect of the independent variables are called dependent variables. These all values are contrasted to the control group with a fixed value.
Therefore, in option B, the variables are the characteristics with the same or different values.
Learn more about variables here:
https://brainly.com/question/13760390
#SPJ2
what is the nominal mass of adenosine? answer should be in amu but written only as a number without ""amu"" included.
Answers
Adenosine has a nominal mass of 267. The mass of a molecule is equal to the sum of its constituent atoms' atomic masses. Adenine molecules are joined to ribose sugar molecules to form the nucleoside known as adenosine.
Ribose has an atomic mass of 132.0 amu, while adenine is 135.0 amu in size. As a result, adenosine has a total nominal mass of 267 amu. By transporting adenine nucleotides, which are involved in the transfer of energy in the form of adenosine triphosphate (ATP), adenosine serves to control the energy generation in cells.
Adenosine also has a role in a variety of biological processes, including cell differentiation, signal transduction, and gene expression. The control of the cardiovascular system depends on adenosine.
Learn more about Ribose at:
https://brainly.com/question/31817478
#SPJ1
If I initally have a gas at a pressure of 12 atm, a volume of 23 L, and a temperature of 200 K, and then I raise the pressure to 14 atm and increase the temperature to 300 K, what is the new volume of the gas?
Answers
Answer: 30 L
Explanation: Use the combined gas law: P1V1/T1 = P2V2/T2
We want V2, so rearrange:
V2 = V1(T2/T1)(P1/P2)
Note how I've grouped the temperature and pressure into ratios. This allows us to cancle those units quickly and gives a perspective on what we should expect. Enter the data:
V2 = (23L)*(0.6667)*(0.8571)
V2 = 29.6 L 30 L for 2 sig figs
which period of the periodic table is completely made up of elements with no known stable ions?
Answers
Answer:
I think the answer is period 7
Explanation:
The period in periodic table, in which no stable ions of elements exists is 7th period. They contains actinides.
What are actinides?Actinides are 7th period elements classified to F block of periodic table. A periodic table contains vertical columns called groups and horizontal rows called periods. Each elements are classified into suitable groups based on their similarity in properties with other group elements.
There are four blocks in periodic table which are s, p, d and f block. S block elements are metals and d block contains transition metals and p block have non metals including gases and metalloids.
Actinides are f- block metals. All the elements in the period are radioactive. That's why they don't have a stable isotope or ion. They continuously undergo radioactive decay by the emission of charged particles.
Hence, the period containing unstable elements is 7th one.
To find more about actinides, refer the link below:
https://brainly.com/question/5188867
#SPJ2
2) A sealed metal cylinder with a gas in it is thrown into a fire.
a) Did this increase or decrease the pressure of the gas in the cylinder?
b) Explain, in terms of speed of the gas particles and particle collisions against the cylinder walls, why the pressure changes the way it does.
Answers
Answer:The kinetic energy will increase and the pressure of the gas will also increase.
Explanation:
An increase in temperature will normally cause an increase in volume. Because the gas is enclosed in a rigid container the volume of the container can not increase.
An increase in temperature is by definition is an increase in kinetic energy. The increase in kinetic energy will cause the molecules of the gas to move more rapidly. As the molecules move more rapidly they collide with each other more violently. These collisions will cause an increase collisions will cause an increase in pressure.
a. Ammonia is made by the reaction of nitrogen with hydrogen.
N₂ + 3 H2 → 2 NH3
Calculate the mass of ammonia that can be formed when 100 g of nitrogen reacts with an excess of hydrogen.
Answers
The mass of ammonia obtained is 128.8 g
What is molar ratio?A molar ratio is the ratio between the molar amounts of any two compounds that participate in a balanced chemical reaction. A chemical equilibrium equation provides a comparison of the ratios of molecules required to complete a reaction. It is not possible to calculate the molar ratio of the imbalanced formula.
N₂ + 3H₂ → 2NH₃
The molar ratio of N₂ and NH₃ is 1:2, which means that every mole of nitrogen produces two moles of ammonia.
Moles of nitrogen = 100/28
= 3.57 moles
So, the moles of ammonia will be = 2 × 3.57
= 7.14 moles
Now, to determine the mass:
Moles of ammonia = mass of ammonia produced/molar mass of ammonia
7.14 = m/18.04
m = 128.8 g
To know more about mole ratio, visit:
https://brainly.com/question/15288923
#SPJ1