9. A student needs to prepare 100 mL of a 0.50 M ammonium chloride, NH4Cl,
solution. How much solute does he need?
Answers
Answer:
2.67g of NH4Cl are required
Explanation:
Molarity is an unit of concentration in chemistry defined as the moles of solute (In this case, NH4Cl), per liter of solution.
To prepare 100mL of a 0.50M are required:
100mL = 0.100L * (0.50 moles / L) = 0.0500 moles NH4Cl
As molar mass of NH4Cl is 53.491g/mol:
0.0500 moles * (53.491g / mol) =
2.67g of NH4Cl are requiredRelated Questions
Question 28 Marks: 1 When chlorine is added to waterChoose one answer. a. chlorine gas is formed b. HOCl is considered the primary product c. HCl is considered the primary product d. ozone is produced in large quantities
Answers
Answer: HOCl is considered the primary product when chlorine is added to water.
When chlorine is added to water, it reacts with water to form a mixture of hypochlorous acid (HOCl) and hydrochloric acid (HCl) as the primary products. The exact ratio of these products depends on the pH of the water. In acidic solutions, more HCl is formed, while in basic solutions, more HOCl is formed. HOCl is a powerful disinfectant and is commonly used in water treatment to kill bacteria, viruses, and other harmful microorganisms. It works by disrupting the cell membranes of these microorganisms, which causes them to die. However, HOCl can also react with organic compounds in the water to form harmful byproducts, such as trihalomethanes, which can pose a health risk. To minimize the formation of harmful byproducts, water treatment plants carefully control the amount of chlorine added to the water and adjust the pH of the water to optimize the formation of HOCl. Overall, the addition of chlorine to water is an important step in ensuring safe and clean drinking water for the public.
Learn more about HOCl here:
https://brainly.com/question/9440783
#SPJ11
consider the unbalanced redox reaction: mno−4(aq)+zn(s)→mn2+(aq)+zn2+(aq)
Answers
The balanced equation for the given redox reaction is:
2MnO4-(aq) + Zn(s) + 8H+(aq) → 2Mn2+(aq) + Zn2+(aq) + 4H2O(l)
The unbalanced redox reaction given is:
MnO4-(aq) + Zn(s) → Mn2+(aq) + Zn2+(aq)
In order to balance the redox reaction, we need to ensure that the number of atoms and charges on both sides of the equation are equal. Let's break down the reaction and balance it step by step.
First, let's balance the atoms other than oxygen and hydrogen. We have one manganese (Mn) atom on the left side and one on the right side, so the number of Mn atoms is already balanced. Similarly, we have one zinc (Zn) atom on each side, which is also balanced.
Next, let's balance the oxygen atoms. On the left side, we have four oxygen (O) atoms in the MnO4- ion, while on the right side, we have two oxygen atoms in the Mn2+ ion. To balance the oxygen atoms, we need to add two water (H2O) molecules on the right side.
Now, let's balance the hydrogen (H) atoms. On the left side, there are no hydrogen atoms, while on the right side, we have four hydrogen atoms in the two water molecules we added earlier. To balance the hydrogen atoms, we need to add four hydrogen ions (H+) on the left side.
Finally, let's balance the charges. On the left side, the overall charge is -1 from the MnO4- ion, while on the right side, the overall charge is +2 from the Mn2+ ion and +2 from the Zn2+ ion. To balance the charges, we need to add two electrons (e-) on the left side.
The balanced equation for the given redox reaction is:
2MnO4-(aq) + Zn(s) + 8H+(aq) → 2Mn2+(aq) + Zn2+(aq) + 4H2O(l)
In this balanced equation, both the number of atoms and charges are equal on both sides, satisfying the law of conservation of mass and charge.
Learn more about redox reactions :
https://brainly.com/question/28300253
#SPJ11
What location of an atom are protons,neutrons, and electrons found
Answers
Answer:
Nucleus is the answer of your question
Answer:
nucleus is your awnser
Describe how you think rock outcrops and exposures in southwestern U.S.A would look in comparison with rocks in northeastern U.S.A.
Answers
Answer:
Generally speaking, rocks in the southwestern U.S.A tend to be much older and more eroded than those found in the northeast. The rock outcrops and exposures in this region will likely consist of sedimentary layers that have been exposed due to erosion over time, as well as some ancient igneous intrusions such as granite or basalt. In comparison, the northeastern U.S.A tends to contain younger rocks with fewer signs of weathering and erosion; these outcrops and exposures may include a variety of metamorphic rocks such as slate or gneiss along with granites from nearby mountain ranges like the Appalachians or Adirondacks.
A ball is rolling 0. 25 meters per second. How fast is it moving in units of miles per hour?
Answers
Answer:
0.56 mi/hr
Explanation:
hope this helps
What properties of solid naoh necessitate standardization of a naoh solution?
Answers
The properties of solid NaOH that necessitate standardization of a NaOH solution are primarily its Hygroscopic nature, its tendency to react with atmospheric CO2, and its variable purity.
1. Hygroscopic nature: Solid NaOH (sodium hydroxide) has a strong affinity for water, meaning it readily absorbs moisture from the air. This can lead to changes in its mass and concentration, affecting the accuracy of a prepared NaOH solution.
2. Reaction with atmospheric CO2: Solid NaOH reacts with carbon dioxide (CO2) present in the air, forming sodium carbonate (Na2CO3). This reaction alters the composition of the NaOH, again affecting its concentration in a solution.
3. Variable purity: Commercially available NaOH often contains impurities due to the manufacturing process. These impurities can impact the effective concentration of NaOH in a solution.
To ensure accurate results in experiments, it's crucial to standardize the NaOH solution. Standardization is the process of determining the exact concentration of a solution using a primary standard, such as potassium hydrogen phthalate (KHP).
By titrating the NaOH solution with a known amount of primary standard, you can precisely calculate its concentration, accounting for any changes caused by its hygroscopic nature, reaction with atmospheric CO2, and impurities.
To Learn More About Hygroscopic
https://brainly.com/question/29493408
#SPJ11
Determine whether the following closed-loop transfer functions for (Y/Ysp) are stable or unstable or underdetermined (requires further analysis), provide your evidences (30 Points): 8Kc a 5S+1 8Kc b) S2+3S+2 8Kc c S3+6S2+12S+8+8Kc
Answers
To determine the stability of the given closed-loop transfer functions, we need to analyze the locations of the poles in the complex plane. If all the poles have negative real parts, the system is stable.
If any pole has a positive real part, the system is unstable. If there are poles on the imaginary axis or have zero real parts, further analysis is required.
a) Transfer function: 8Kc / (5S + 1)
The pole of this transfer function is at S = -1/5. Since the real part of the pole is negative, the system is stable.
b) Transfer function: 8Kc / (S^2 + 3S + 2)
The poles of this transfer function are at S = -1 and S = -2. Both poles have negative real parts, so the system is stable.
c) Transfer function: 8Kc / (S^3 + 6S^2 + 12S + 8 + 8Kc)
To determine the stability of this transfer function, we need to find the roots of the polynomial in the denominator. The characteristic equation is S^3 + 6S^2 + 12S + 8 + 8Kc = 0.
By analyzing the roots of the characteristic equation for different values of Kc, we can determine the stability. If all the roots have negative real parts for any value of Kc, the system is stable.
However, without a specific value for Kc provided, we cannot conclusively determine the stability of the system. The stability of the system will depend on the specific value of Kc and the locations of the roots of the characteristic equation. Further analysis is required to determine the stability.
In summary:
a) The system is stable.
b) The system is stable.
c) The stability requires further analysis as it depends on the specific value of Kc and the locations of the roots of the characteristic equation.
learn more about closed-loop here
https://brainly.com/question/11995211
#SPJ11
write the empirical formula for C8H16O2
Answers
Answer:
C4H802
Explanation:
Empirical formula is the simplest formula so you break the compound to its simplest form
If it was helpful kindly follow
What is the missing word. Please Help Me. Thank you!!
Before adding to the cell, aluminium oxide is mixed with _____________ to lower its melting point.
Answers
Hope this helped =D
Answers to 33-38 pls

Answers
Answer:
-5
Explanation:
For what I did was:
Switch the numbers
(35-33)
Do the math
Then add a negative because the smaller number is first meaning it will be a negative number
A chemist designs a galvanic cell that uses these two half-reactions: 2h2o 2e h2 2)h
Answers
The galvanic cell design involves the reduction of hydrogen ions to hydrogen gas at the cathode and the oxidation of hydrogen gas to hydrogen ions at the anode, resulting in a transfer of electrons and the overall reaction of 2H₂O + H₂ → 2H⁺ + 2OH⁻ + H₂.
The two half-reactions provided are:
1. 2H₂O → 2H⁺ + 2e⁻ (reduction half-reaction)
2. H₂ → 2H⁺ + 2e⁻ (oxidation half-reaction)
To design a galvanic cell, we need to combine these half-reactions in such a way that the reduction and oxidation reactions occur separately. This can be achieved by connecting the two half-cells with a salt bridge or a porous membrane.
In this case, the reduction half-reaction involves the reduction of water (H₂O) to hydrogen gas (H₂) by gaining two electrons (2e⁻). The oxidation half-reaction involves the oxidation of hydrogen gas (H₂) to hydronium ions (H⁺) by losing two electrons (2e⁻).
To construct the galvanic cell, we would typically represent the anode (oxidation) and cathode (reduction) compartments. The anode is where oxidation occurs, and the cathode is where reduction occurs. The half-cell notation for each half-reaction would look like this:
Anode (oxidation half-reaction): H₂ → 2H⁺ + 2e⁻
Cathode (reduction half-reaction): 2H₂O + 2e⁻ → 2H₂ + 2OH⁻
Overall, the balanced reaction of the galvanic cell would be:
2H₂O + H₂ → 2H⁺ + 2OH⁻ + H₂
This reaction represents the transfer of electrons from the anode to the cathode, with hydrogen ions (H⁺) being reduced to form hydrogen gas (H₂) at the cathode, and hydrogen gas (H₂) being oxidized to form hydrogen ions (H⁺) at the anode.
Learn more about galvanic cell here:
https://brainly.com/question/15096829
#SPJ4
say hiii for the camera
Answers
Answer:
Hello?
Explanation:
Answer:
hiiiiii camreaaaaaa. also hows youre dayy
Explanation:
hi :]
The theory of plate tectonics states that the EEHLISRTOPH ______________ is
divided into pieces that slowly move on top of the AEEENOSHPTSHR
______________________.
Answers
Answer:
n nnjhuig
Explanation:
bjbhvucvcg
A cake recipe calls for 0.75 kg of butter. How many grams of butter would be needed for 4 cakes
Answers
Answer:
So if you divide the kg of butter and the cake that will be your answer and then you should have a fraction so the fraction would be 0.25 over 2 because you simplified it. 0.25/2
Explanation:
Fill The Blank! there are _____ specific amino acids required in the diet daily
Answers
There are nine specific amino acids required in the diet daily.
The building blocks of proteins, known as amino acids, are crucial for the proper operation of cells in living things. Twenty different types of amino acids, each with a distinct chemical structure and side chain, are frequently present in proteins. Nine of these 20 amino acids are regarded as essential amino acids, which means that the body cannot produce them and that they must be received from food. Together with histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine, these amino acids are considered to be essential. Reduced immunological function, muscle atrophy, and poor growth and development are just a few health issues that can arise from inadequate intake of these critical amino acids.
Learn more about amino acids here:
https://brainly.com/question/13943912
#SPJ4
A company desires to produce nickel from refining nickel oxide and sulfide ores. The first step of the process will produce a nickel carbonyl gas, Ni(CO)4, by heating the ore. The second step is as follows: Ni(CO)4(g) Ni(s) + 4CO(g) What effect on the process would be caused by increasing pressure? Ni would be produced at a higher rate. Ni would be produced at a lower rate. There would be no effect on this particular reaction.
Answers
Increasing the pressure in the system would favor the forward reaction in the equilibrium, which is the production of Ni and CO gas.
According to Le Chatelier's principle, when a system at equilibrium is subjected to a change in pressure, it will shift to the direction that reduces the effect of the change.
In this case, increasing the pressure would cause the system to shift towards the side with fewer moles of gas, which is the side with solid Ni and CO gas.
Therefore, increasing the pressure would result in a higher rate of nickel production, as more Ni(CO)4 would be converted to Ni and CO gas. However, it is important to note that increasing the pressure beyond a certain point may not result in any significant changes in the rate of the reaction, as the equilibrium constant for this reaction may be reached at high pressures.
Learn more about equilibrium here:
https://brainly.com/question/30807709
#SPJ1
why is edta used to determine the hardness of water
Answers
EDTA (ethylenediaminetetraacetic acid) is commonly used to determine the hardness of water due to its ability to form complexes with metal ions, particularly calcium and magnesium ions.
Water hardness refers to the concentration of calcium and magnesium ions present in water. These ions can cause scaling, reduce the effectiveness of soaps, and have other negative effects. EDTA acts as a chelating agent, meaning it can bind to metal ions and form stable complexes.
In the process of determining water hardness, a known amount of EDTA solution is added to a water sample. The EDTA molecules form complexes with the calcium and magnesium ions present in the water.
The endpoint of the titration is reached when all the metal ions are complexed by the EDTA, resulting in a color change or an indicator reaching a specific endpoint.
To know more about hardness of water refer:
https://brainly.com/question/28178305
#SPJ11
How are the atomic number and the number of protons related to each other?
Answers
what is the third quantum number of a 3 s 2 electron in phosphorus, 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3 ?
Answers
The third quantum number (m_l) of a 3s² electron in phosphorus is 0.
The third quantum number, denoted as m_l, represents the magnetic quantum number and describes the orientation of an orbital within a subshell. It can have integer values ranging from -l to +l, where l is the azimuthal quantum number.
In the electron configuration of phosphorus, we see that the 3s subshell is being filled. The azimuthal quantum number (l) for the 3s subshell is 0. Since the electron is in the 3s² subshell, there are two electrons present in the 3s orbital.
For the two electrons in the 3s orbital, they will have opposite spins due to the Pauli exclusion principle. However, the magnetic quantum number (m_l) for both electrons in the 3s orbital will be the same, which is 0.
Therefore, the third quantum number (m_l) of a 3s² electron in phosphorus is 0. This means that both electrons in the 3s orbital have the same orientation within the subshell.
To learn more about quantum number, here
https://brainly.com/question/31955577
#SPJ4
The following properties are either physical or chemical. Which one is different from the rest based on those two categories?
Answers
Answer:
Boiling point: physical
Density: physical
Ductility: physical
Heat of combustion: chemical
Explanation:
Chemical properties can only be analyzed by putting the substance through a chemical reaction, such as combustion.
Physical properties can be analyzed without altering the substance chemically. With that in mind:
Boiling point is the physical property, density is the physical property, ductility is also a physical property but combustion of heat is a chemical property.
What is physical and chemical property?A physical property is the characteristic of a substance or compound that is observed without any change in the identity of the particular substance.
The physical properties of a substance is its colour, hardness, density, and melting as well as boiling points.
A physical property is a characteristic of matter that involves in physical change and physical changes are reversible and there is no production of any new substance.
The chemical change is the property of a substance due to which new substances are formed and it is non reversible change.
The chemical properties are flammability, toxicity, heat of combustion, coordination number, enthalpy of formation, and oxidation states.
Therefore, Boiling point is the physical property, density is the physical property, ductility is also a physical property but combustion of heat is a chemical property.
Learn more about physical and chemical property here:
https://brainly.com/question/1935242
#SPJ2
What does the slope of a distance versus time graph represent?
Total time
Average time
Average distance
Speed
Answers
Answer:
Speed
Explanation:
Motion can be represented by a distance-time graph, which plots distance on the y-axis and time on the x-axis. The slope of a distance-time graph represents speed. The steeper the slope is, the faster the speed.
Hope this helps!:)
what is the mass in grams of 0.00125 mole of sodium Na=23
Answers
Answer:
0.03g
Explanation:
Given parameters:
Number of moles of Na = 0.00125mole
Unknown:
Mass in grams of Na = ?
Solution:
To find the mass of this substance, we simply multiply the number of moles with the given molar mass of Na.
Mass of Na = number of moles x molar mass
Mass of Na = 0.00125 x 23 = 0.03g
Which of the following combinations can be used to make a buffer? (Assume equal
volumes are used.)
A) 0.20 M NH, and 0.20 M HCI
B) 0.20 M NH, and 0.10 M NH CI
C) 0.20 M NH, and 0.10 M HF
D) 0.10 M NH CI and 0.10 M NaF
Answers
0.20 M NH₄, and 0.20 M HCI and 0.20 M NH₄, and 0.10 M HF are combinations that can be used to make a buffer. Thus option A and C are correct.
A buffer solution is an acid or a base aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa.Its pH changes very little when a small amount of strong acid or base is added to it.
Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean.
As the combination of HCl and NH₄, and NH₄ and HF are combinations that can be used to make a buffer. Thus option A and C are correct.
Learn more about buffer,here:
https://brainly.com/question/31847096
#SPJ12
Balance the following chemical equation:
C2H60+02 → CO2 + H20
Answers
Answer:
Explain: In order to balance the chemical equation, you need to make sure the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side. In order make both sides equal, you will need to multiply the number of atoms in each element until both sides are equal.
2. How many moles of hydrogen are produced from the reaction of 3.86 moles of zinc with an excess of
hydrochloric acid?
Answers
Answer:
as per given balanced equation one mole Zn produces 1mole Hydrogen
when reacted with excess acid.
So 3 moles Zn will give 3moles Hydrogen
Explanation:
According to the balanced chemical equation, one mole of Zn produces one mole of hydrogen . Therefore, 3.86 moles of Zn metal will produce 3.86 moles of hydrogen gas.
What is mole ratio ?Mole ratio refers to the ratio of the number of moles of one substance in a chemical reaction to the number of moles of another substance in the same reaction.
It is derived from the balanced chemical equation for the reaction, which indicates the relative amounts of each substance involved in the reaction.
Mole ratio is important in stoichiometry, which is the study of the quantitative relationships between reactants and products in a chemical reaction.
The given chemical reaction is written as follows:
Zn + 2 HCl ⇒ ZnCl₂ + H₂
According to the balanced chemical equation, one mole of Zn produces one mole of hydrogen . Therefore, 3.86 moles of Zn metal will produce 3.86 moles of hydrogen gas.
Find more on mole ratio :
https://brainly.com/question/15288923
#SPJ2
Ammonium (NH4+), carbonate (CO32−), and phosphate (PO43−) are all examples of: (a) multivalent metals (b) polyatomic ions (c) covalent molecules (d) molecular compounds. Help pleasee
Answers
Answer:
Option b) polyatomic ions
Explanation:
Polyatomic ions are ions consisting of two or more atoms.
From the question given above, we can see that each ions consist of more than one atom as shown below:
Ions >>>>>> Number of atom present
NH4+ >>>>> 2
CO32− >>>> 2
PO43− >>>> 2
Thus, we can say that the above ions are polyatomic ions.
Answer:
It's C.
Explanation:
54.0 g Al reacts with 64.0 g O2 to
form Al2O3 according to the equation.
4A1+30₂ → 2Al2O3
Al: 26.98 g/mol Al2O3: 101.96 g/mol
How many grams of Al2O3 form
from 54.0 g Al?
[?] g Al₂O3

Answers
Answer:
102 g Al₂O₃
Explanation:
To find the mass of Al₂O₃, you need to (1) convert grams Al to moles Al (via molar mass), then (2) convert moles Al to moles Al₂O₃ (via mole-to-mole ratio from reaction coefficients), and then (3) convert moles Al₂O₃ to grams Al₂O₃ (via molar mass). It is important to arrange the ratios in a way that allows for the cancellation of units. The final answer should have 3 sig figs to reflect the given values.
Molar Mass (Al): 26.98 g/mol
Molar Mass (Al₂O₃): 101.96 g/mol
4 Al + 3 O₂ ---> 2 Al₂O₃
54.0 g Al 1 mole 2 moles Al₂O₃ 101.96 g
-------------- x ----------------- x ----------------------- x ---------------- = 102 g Al₂O₃
26.98 g 4 moles Al 1 mole
Answer: 102 for 54.0 and 136 for 64.0
Explanation:
How much heat is added if 0.2067g of water is increased in temperature by 0.855
degrees C?
Answers
Answer:
0.756 J
Explanation:
Temperature change (delta T) = 0.855 degrees Celsius
Mass of water (m) = 0.2067 g
Specific heat capacity of water (c) = 4.28 J/g degrees C
Heat added = m * c * delta T
= (0.2067 * 4.28 * 0.855) J
= 0.756 J
Which of these is a reason why nuclear power is important?
4.4.2 nuclear power
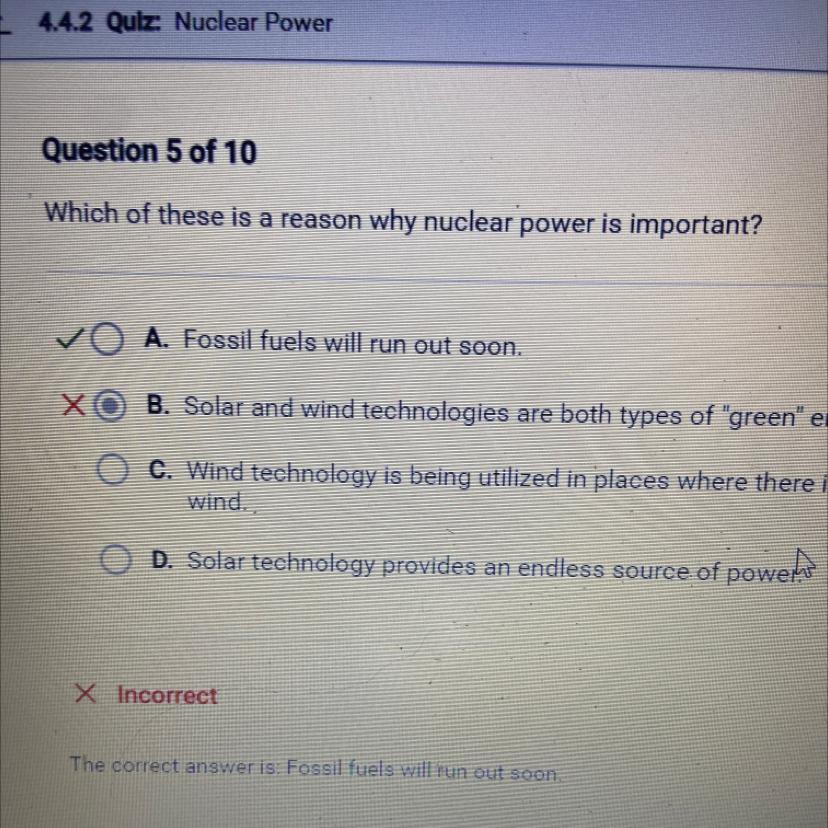
Answers
Answer:
Fossil fuels will run out soonWhat is the universal solvent?
A. hydrogen
B. water
C. oil
D. acid
Answers
Answer:
water
Explanation:
because it's capable of dissolving more substances
Answer:
B. water It can dissolve more substances
Explanation: