6h2o + 6cl2 = 2hclo3 + 10hcl for each of the following equaiton identify what is oxidized what is redeuced, the oxidizing agent and the reducing agent
Answers
In the equation 6H2O + 6Cl2 = 2HClO3 + 10HCl, the species being oxidized is Cl2, and the species being reduced is H2O. The oxidizing agent is H2O, and the reducing agent is Cl2.
6H2O + 6Cl2 = 2HClO3 + 10HClOxidation is the process of losing electrons. Reduction is the process of gaining electrons.In the given chemical equation, H2O (water) is oxidized and Cl2 is reduced. In this reaction, Chlorine gets oxidized and water gets reduced.Oxidized substance: H2OReduced substance: Cl2Oxidizing agent: Cl2Reducing agent: H2OExplanation:Water is the reducing agent since it is being oxidized. Chlorine is the oxidizing agent since it is being reduced. Chlorine oxidizes water, and it is reduced from Cl2 to HCl. Water reduces chlorine, and it is oxidized from H2O to HClO3.
Know more about Oxidization here:
https://brainly.com/question/9496279
#SPJ11
Identify what is oxidized and what is reduced, the oxidizing agent and the reducing agent in the following equation
6h2o + 6cl2 = 2hclo3 + 10hcl
Related Questions
Which term is defined as the region in an atom where an electron is most likely to be
located?
1.
nucleus
2.
orbital
3.
quanta
4.
spectra
Answers
Answer:
"2.Orbital." I think
Explanation:
Risk*
Given subsets A and B of Ω, identify all sets in σ(A,B).
Answers
The sets in σ(A,B) are the smallest σ-algebra that contains both A and B.
In probability theory and measure theory, a σ-algebra is a collection of subsets of a given set Ω that satisfies certain properties. The notation σ(A,B) represents the smallest σ-algebra that contains both subsets A and B. This means that σ(A,B) consists of all possible subsets that can be formed by taking the union, intersection, and complement of sets in A and B.
To understand this concept better, let's consider an example. Suppose we have a set Ω = {1, 2, 3, 4} and two subsets A = {1, 2} and B = {2, 3}. The σ-algebra σ(A,B) would include the empty set, the set Ω itself, as well as other subsets such as {1}, {2}, {3}, {1, 2}, {2, 3}, and {1, 2, 3}. It would also include their complements, for example, the complement of {1} would be {2, 3, 4}.
The σ-algebra σ(A,B) is important in probability theory as it allows us to define probability measures and study various properties of events and random variables. By identifying all the sets in σ(A,B), we can determine the range of events that can be analyzed within this framework.
Learn more about σ-algebras
brainly.com/question/32708586
#SPJ11
what is a property of every mixture
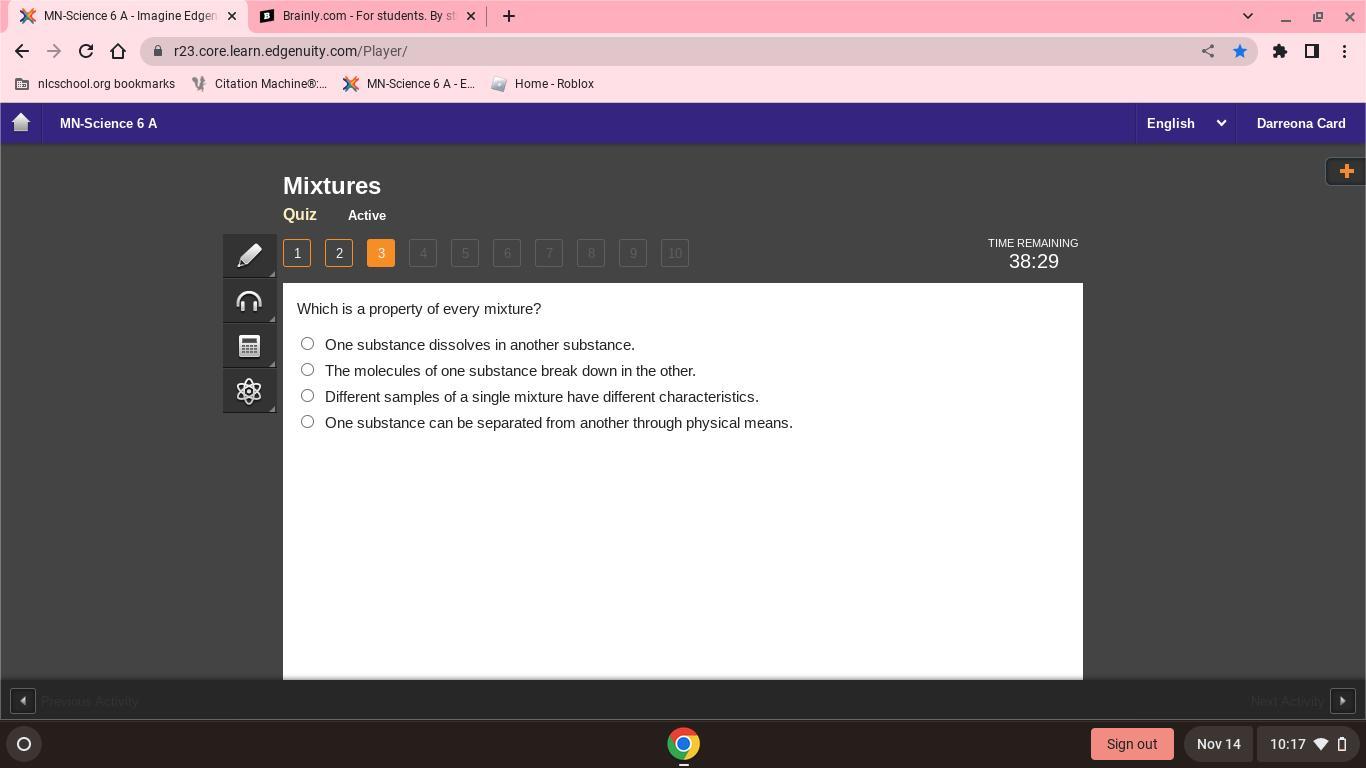
Answers
The property of every mixture is right response is D)One substance can be separated from another through physical means.
A mixture is a combination of two or more substances that are physically merged or mingled without losing their own identities.This indicates that the combination does not alter chemically and can be physically separated (like filtration). It may contain molecules that are solid, liquid, or gaseous.Homogeneous mixtures (having a consistent composition, so that every sample will have the same attribute) and heterogeneous mixtures are the two main categories of mixtures ( non uniform composition that is not every sample will have same property).As a result, D) is a characteristic of every blend.To learn more about mixture visit:
brainly.com/question/24898889
#SPJ1
Molarity of Kool Aid solutions can be calculated by comparing the concentrations of Kool Aid powder and sugar added to a given volume of water. The molar mass of Kool Aid will be the same as that of sugar for our purpose. The molecular formula for sugar is C12H22O11- Your objective for this lab will be to calculate the molarity of Kool Aid desired based on package directions. You will then be provided two concentrated Kool Aid solutions. You will use dilution calculations to determine the amount of water and concentrated solution you will need in order to prepare 65 mL of the desired molarity.
Calculate the molarity of Kool Aid desired based on the following information from the package directions.
1 package Kool Aid powder = 4. 25 grams 1 cup sugar = 192. 00 grams
2. 00 quarts of water (1. 06 quarts = 1 liter)
Answers
The amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
To calculate the molarity of Kool Aid desired, we need to determine the number of moles of Kool Aid powder and sugar in the package. Since the molecular formula for sugar is C12H22O11, we can calculate its molar mass as follows:
Molar mass of C12H22O11 = (12 * 12.01) + (22 * 1.01) + (11 * 16.00)
= 144.12 + 22.22 + 176.00
= 342.34 g/mol
Given that the package contains 4.25 grams of Kool Aid powder, we can calculate the number of moles of Kool Aid powder using its molar mass:
Number of moles of Kool Aid powder = Mass / Molar mass
= 4.25 g / 342.34 g/mol
≈ 0.0124 mol
Similarly, for the sugar, which has a molar mass of 342.34 g/mol, we can calculate the number of moles of sugar using its mass:
Number of moles of sugar = Mass / Molar mass
= 192.00 g / 342.34 g/mol
≈ 0.5612 mol
Now, to calculate the molarity of the desired Kool Aid solution, we need to determine the volume of water. Given that 1.06 quarts is equal to 1 liter, and we have 2.00 quarts of water, we can convert it to liters as follows:
Volume of water = 2.00 quarts * (1.06 liters / 1 quart)
= 2.12 liters
To find the molarity, we use the formula:
Molarity (M) = Number of moles / Volume (in liters)
Molarity of Kool Aid desired = (0.0124 mol + 0.5612 mol) / 2.12 L
≈ 0.286 M
To prepare 65 mL of the desired molarity, we can use dilution calculations. We need to determine the volume of concentrated solution and the volume of water needed.
Let's assume the concentration of the concentrated Kool Aid solution is C M. Using the dilution formula:
(C1)(V1) = (C2)(V2)where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Given that C1 = C M and V1 = V mL, and we want to prepare a final volume of 65 mL (V2 = 65 mL) with a final concentration of 0.286 M (C2 = 0.286 M), we can rearrange the formula to solve for the volume of the concentrated solution:
(C M)(V mL) = (0.286 M)(65 mL)
V mL = (0.286 M)(65 mL) / C M
So, the amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
For more such questions on concentrated visit:
https://brainly.com/question/28564792
#SPJ8
has any one done 2020 chemistry paper 1 aqa gcse
Answers
Answer:
what do you mean?
Explanation:
the solubility of cui is 2 x 10¯6 molar. what is the solubility product constant, ksp, for cui?
Answers
The solubility product constant for CuI where solubility of CuI is \(2*10^{-6}\) molar is \(4 * 10^{-12}\).
The solubility of CuI is 2 x 10^-6 M. To find the solubility product constant (Ksp) for CuI, we need to consider its dissociation in water:
CuI(s) ⇌ Cu⁺(aq) + I⁻(aq)
Since the solubility is 2 x 10^-6 M, the concentrations of both Cu⁺ and I⁻ ions are equal to\(2*10^{-6}\) M. The Ksp is calculated as the product of the concentrations of the ions:
Ksp = [Cu⁺] * [I⁻]
Substitute the given solubility values:
\(Ksp = (2 *10^-6) * (2 *10^-6)\)
\(Ksp = 4 * 10^{-12}\)
So, the solubility product constant (Ksp) for CuI is \(4 * 10^{-12.\)
Learn more about solubility product constant here:
https://brainly.com/question/1419865
#SPJ11
During an exothermic reaction, H for the reactants was 890 kJ/mol. Which of the following statements is correct about the H for the products and the comparison of the energy in bonds? (5 points) Select one: a. It is less than 890 kJ/mol, and the amount of energy required to break bonds is greater than the amount of energy released in forming bonds. b. It is less than 890 kJ/mol, and the amount of energy required to break bonds is less than the amount of energy released in forming bonds. c. It is greater than 890 kJ/mol, and the amount of energy required to break bonds is greater than the amount of energy released in forming bonds. d. It is greater than 890 kJ/mol, and the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
Answers
Answer:
The answer is b. It is less than 890 kJ/mol, and the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
Explanation:
i just took the test sorry i could not help you before but i hope this helps someone!
The statement which is correct about H for the products and the comparison of the energy in bonds is that the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
What is a Bond?This is defined as an attraction between atoms, molecules etc which enables the formation of chemical compounds.
Since is less than 890 kJ/mol , the amount of energy required to break bonds is less than the amount of energy released in forming bonds thereby making option A the most appropriate choice.
Read more about Bond here https://brainly.com/question/819068
lewis structure of socl2 is given. explain in words how hybrid orbitals are formed on s atom by first drawing the orbital diagram of s (only show vale
Answers
To explain how hybrid orbitals are formed on the sulfur (S) atom in SOCl2, we need to first draw the orbital diagram of S, focusing on the valence electrons.
Sulfur (S) has the electron configuration [Ne] 3s2 3p4. The valence shell of sulfur consists of 6 electrons, with 2 in the 3s orbital and 4 in the 3p orbitals. In order to form hybrid orbitals, the 3s and 3p orbitals of sulfur will combine to create a set of new hybrid orbitals.
To determine the number and type of hybrid orbitals, we count the number of electron groups around the sulfur atom, which includes both lone pairs and bonded atoms. In SOCl2, there are 4 electron groups around sulfur: one sulfur-chlorine double bond, and two lone pairs.
Since there are 4 electron groups, sulfur undergoes sp3 hybridization. This means that the 3s orbital and the 3p orbitals (3px, 3py, and 3pz) will hybridize, resulting in four sp3 hybrid orbitals. These hybrid orbitals are oriented in a tetrahedral arrangement around the sulfur atom.
The new sp3 hybrid orbitals will then form sigma bonds by overlapping with the appropriate atomic orbitals of the surrounding atoms (oxygen and chlorine) in the molecule SOCl2. This allows for the formation of the molecular structure and the bonding in the compound.
To learn more about orbitals click here:
brainly.com/question/2321359
#SPJ11
1. explain how u would test the presence of oxygen and hydrogen gases
2. explain how u can get the most accurate reading in titration
3. why is scientific method important in chemistry? state your opinion
4. you are given a bucket of ice cubes, a little sugar, a measuring cylinder and a stopwatch.Plan a experiment to determine whetther sugar increases the melting rate of ice
Answers
sorry
i don’t know but maybe next time
the acid catalyzed hydrolysis of the ester, ch3ch2cooch3 will yield which one classification of a compound?
Answers
It will yield ethanol (CH3CH2OH) and acetic acid (CH3COOH). Ethanol is an alcohol, and acetic acid is a carboxylic acid.
Overall reaction: CH3CH2COOCH3 + H2O → CH3CH2OH + CH3COOH The acid-catalyzed hydrolysis of the ester CH3CH2COOCH3 (ethyl acetate) will yield an alcohol and a carboxylic acid. Specifically, it will yield ethanol (CH3CH2OH) and acetic acid (CH3COOH). In this reaction, the ester is broken down by the addition of water in the presence of an acid catalyst, which provides a proton to facilitate the reaction. The ester bond is cleaved, resulting in the formation of the alcohol and the carboxylic acid.
Learn more about catalyzed hydrolysis here:
https://brainly.com/question/31035725
#SPJ11
There are no attractive or repulsive forces between gas molecules. How does that affect the motion of gas particles?
Answers
The absence of attractive or repulsive forces between gas molecules means that they are free to move independently and randomly. This results in the motion of gas particles being characterized by constant collisions and changes in direction and speed. Without any forces to constrain their movement, gas particles will continue to move until they collide with other particles or the walls of their container. This is what causes gases to fill up any container they are in, as their independent motion allows them to spread out evenly throughout the available space.
What is attractive force?
An attractive force is a force that pulls or draws two or more objects or particles towards each other. It is the opposite of a repulsive force, which pushes objects or particles away from each other.
Attractive forces can be observed in a variety of contexts, including gravity, electromagnetism, and intermolecular forces in chemistry. For example, the force of gravity between two objects is an attractive force that pulls them together, while the electromagnetic force between opposite charges is also an attractive force.
What is repulsive force?
A repulsive force is a force that pushes two or more objects or particles away from each other. It is the opposite of an attractive force, which pulls objects or particles towards each other.
Repulsive forces can be observed in a variety of contexts, including electromagnetism and intermolecular forces in chemistry. For example, the force between two like charges is repulsive, while the force between two like magnetic poles is also repulsive.
To know more about Gas molecules:
https://brainly.com/question/30597086
#SPJ11
Given the system at equilibrium:
H3PO4 + 3 H2O <-----> 3 H3O+ + PO4^3-
If Na3PO4(s) is added, there will be a decrease in the
concentration of
A) Na+
B) PO4^3–
C) H3O+
D) H2O
Answers
Answer:
Adding Na3PO4(s) will introduce more PO4^3- ions into the solution, which will react with H3O+ ions to form more H3PO4 and H2O through the reverse reaction. This will shift the equilibrium to the left, decreasing the concentration of H3O+ ions and increasing the concentration of H3PO4 and H2O. Therefore, the concentration of H3O+ ions will decrease, and the correct answer is (C) H3O+.
WILL MARK AS BRAINLIEST IF CORRECT

Answers
Answer:
I don't know what is the actual answer
Find the pH in which the hydrogen ion H+ concentration 6.38×10'-6 moldm'3
Answers
pH = -lg[6.38·10-6] = 5.2
galvanic corrosion can be reduced by avoiding contact between dissimilar metals. t/f
Answers
True. Galvanic corrosion occurs when two dissimilar metals come into contact in the presence of an electrolyte, such as saltwater or acid.
True. Galvanic corrosion occurs when two dissimilar metals come into contact in the presence of an electrolyte, such as saltwater or acid. This creates a flow of electrons between the two metals, causing the more active metal to corrode at an accelerated rate. To prevent galvanic corrosion, it is important to avoid contact between dissimilar metals whenever possible. This can be done by using protective coatings, insulating materials, or by using metals that are similar in their electrochemical properties. In addition, regular maintenance and cleaning can help to prevent the buildup of electrolytes that can lead to galvanic corrosion. Overall, avoiding contact between dissimilar metals is one of the most effective ways to reduce the risk of galvanic corrosion and ensure the longevity of metal structures and equipment.
To know more about Galvanic corrosion visit: https://brainly.com/question/31667168
#SPJ11
PESTLE and SWOT analysis for Harvard case CLEAN EDGE RAZOR: SPLITTING HAIRS IN PRODUCT POSITIONING.
Answers
The PESTLE and SWOT analyses provide a comprehensive assessment of the external and internal factors influencing the Harvard case "Clean Edge Razor: Splitting Hairs in Product Positioning."
The PESTLE analysis examines the external factors that may impact the case. Politically, regulations on product safety and environmental sustainability could affect the razor industry. Economically, consumer purchasing power and economic trends may impact the demand for high-end razors. Sociocultural factors, such as grooming habits and preferences, influence consumer behavior. Technological advancements, such as electric razors and online shopping, pose opportunities and challenges. Legal factors include intellectual property rights and advertising regulations. Environmental considerations involve sustainability and eco-friendly practices.
The SWOT analysis evaluates the internal strengths and weaknesses of the case. Strengths may include the Clean Edge brand reputation and innovative product features. Weaknesses could involve high manufacturing costs or limited market presence. Opportunities may arise from market growth and expanding into new segments. Threats may come from intense competition and changing consumer preferences.
By conducting both analyses, the case can gain insights into the broader industry landscape, identify potential risks and opportunities, and assess its own internal capabilities. This holistic understanding aids in making informed decisions and formulating effective strategies for positioning the Clean Edge Razor product.
Learn more about environmental sustainability here:
https://brainly.com/question/28602390
#SPJ11
(30 points) if anybody sees this can they help me out?
At a depth of 50 m, the pressure is 512 kPa (kilopascals).
A diver breathing air (78% N2, 21% O2) at this depth would have what partial pressure of oxygen in his lungs?
A) 404 kPa
B) 5.1 kPa
C) 108kPa
D) 512 kPa
At 273 K, you collect 0.5 L of oxygen over water that has a total pressure of 22 kPa.
What is the partial pressure of oxygen?
(The partial pressure of water vapor at 273 K is 0.61 kPa .)
A) 36.07 kPa
B) 21.39 kPa
C) 273.61 kPa
D) 22.61 kPa
You breathe in 12.0 L of pure oxygen at 298 K and 1,000 kPa to fill your lungs.
How many moles of oxygen did you take in?
Use the ideal gas law: PV = nRT where R=8.31 L*kPa/mol*K
A) 0.05 mole
B) 1.21 moles
C) 2.42 moles
D) 4.84 moles
Answers
The partial pressure of oxygen in his lungs will be 108 kPa.
The correct option is C.
The partial pressure of oxygen is 21.39 kPa. The correct option is B.
The number of moles of oxygen that you take in is 4.845 moles.
What is the pressure of oxygen in the lungs of the diver?The pressure of oxygen in the lungs of the diver is calculated below:
The Pressure of oxygen = 21 % * 512 kPa
The Pressure of oxygen = 108 kPa.
The partial pressure of oxygen = Total pressure - partial pressure of water vapor
The partial pressure of oxygen = 22 kPa - 0.61 kPa
The partial pressure of oxygen = 21.39 kPa
The number of moles of oxygen taken in, n = PV/RT
n = 1000 * 12 / 8.31 * 298
n = 4.845 moles
Learn more about partial pressure at: https://brainly.com/question/14119417
#SPJ1
Answer:
108 kPa.
Explanation:
What happens in this circuit if one of the light bulbs burns out?
1. The other light bulb will stay on but get dimmer
2. The battery will lose its charge and stop working
3. Both light bulbs will stop glowing
4. The other light bulb will stay on and glow brightly

Answers
Which do you think has the greater total kinetic energy?
a. The molecules of the water in the teacup
b. The molecules of the water in the bathtub
Answers
Answer:
a The molecules of the water in the teacup
hope it helps
How do charged molecules pass through the membrane.
Answers
Answer:
Through transport proteins
Explanation:
There's two transport proteins, channel/pore protein and carrier protein. Charged/polar molecules or ions need specific transport proteins to pass through across the plasma membrane. Transport proteins allows ions/polar molecules to bind to the specific site and allow them to pass through and enter the cell.
(btw, is this a chemistry question? isn't this a biology ques?)
what is the mass number of silver
Answers
Silver (Atomic Mass) :- 107.8682 u
Answer:
Atomic Number: 47
Atomic Mass: 107.8682 u
True or false? The subscripts in a chemical formula do not change for a given compound.
Answers
True. The subscripts in a chemical formula represent the relative number of atoms of each element in a compound. They indicate the ratio of atoms present and remain constant for a given compound.
Changing the subscripts would alter the composition and stoichiometry of the compound.
forces between the negatively charged electron and the positively charged nucleus, allowing the electron to be completely removed from the atom. It is typically measured in units of electron volts (eV) or kilojoules per mole (kJ/mol). Ionization energy is influenced by factors such as the atomic structure, electron shielding, and the effective nuclear charge experienced by the outermost electrons. The ionization energy generally increases as you move across a period in the periodic table due to increased nuclear charge and decreased atomic radius. It also decreases as you move down a group due to increased electron shielding and atomic size. Ionization energy plays a crucial role in understanding chemical reactions, electron configurations, and the reactivity of elements.
Learn more about chemical here:
https://brainly.com/question/29240183
#SPJ11
Which industry would most likely be affected if coal recourses were depleted.
1.Power production
2.Transportation
3.construction
Answers
The size of granules in a sample is 5 micrometers, and
the density is 2 g/mL. Assuming all the granules to be spherical
and the same size, what will be the specific surface area per mL
and per gram. I
Answers
The specific surface area per mL is 251 m²/mL, and the specific surface area per gram is 251 m²/g.
To calculate the specific surface area per mL and per gram accurately, we need to consider the dimensions and units properly.
Given:
Granule size: 5 micrometers
Density: 2 g/mL
First, let's calculate the surface area of a single granule. The surface area of a sphere is given by the formula:
Surface area = 4πr²
where r is the radius of the sphere.
The radius of a granule is half of its diameter, so the radius would be 2.5 micrometers (0.0025 mm).
Surface area of a single granule = 4π(0.0025 mm)² = 4π(6.25 × 10^(-9) mm²) = 3.14 × 10^(-8) mm²
Next, let's calculate the number of granules in 1 mL and 1 gram of the sample.
1 mL of the sample has a volume of 1 mL, and since the density is 2 g/mL, the mass of 1 mL of the sample is 2 grams.
Number of granules in 1 mL = (1 mL / 5 micrometers)^3
= (1 mL / (5 × 10^(-3) mm))^3
= (1 × 10^6 mm³ / (5 × 10^(-3) mm))^3
= (2 × 10^5)^3 = 8 × 10^15 granules
Number of granules in 1 gram = (1 gram / 2 grams) × (1 mL / 5 micrometers)^3
= (1 × 10^3 mm³ / (5 × 10^(-3) mm))^3
= (2 × 10^5)^3
= 8 × 10^15 granules
Finally, we can calculate the specific surface area per mL and per gram:
Specific surface area per mL
= Surface area of a single granule × Number of granules in 1 mL
= 3.14 × 10^(-8) mm² × 8 × 10^15
= 2.51 × 10^8 mm²
Specific surface area per gram = Surface area of a single granule × Number of granules in 1 gram = 3.14 × 10^(-8) mm² × 8 × 10^15 = 2.51 × 10^8 mm²
To convert the specific surface area from mm² to m², we divide by 10^6:
Specific surface area per mL = 2.51 × 10^8 mm² / 10^6 = 251 m²/mL
Specific surface area per gram = 2.51 × 10^8 mm² / 10^6 = 251 m²/g
Therefore, the specific surface area per mL is 251 m²/mL, and the specific surface area per gram is 251 m²/g.
Learn more about surface area from the given link
https://brainly.com/question/951562
#SPJ11
how many moles are there in 4.00 moles of glucose, C₆H₁₂O₆
Answers
2.40 ×10²⁴ molecules are there in 4.00 moles of glucose, C₆H₁₂O₆. A molecule is a collection of at least two atoms.
What is molecule?According on the context, the word can or cannot encompass ions that meet this requirement. A molecule is a collection of at least two atoms bound together by the attractive forces called as chemical bonds.
When speaking of polyatomic ions, the difference between them and ions is frequently ignored in the fields of quantum theory, organic chemistry, especially biochemistry.
number of molecule = number of moles × 6.022×10²³
= 4× 6.022×10²³
= 2.40 ×10²⁴ molecules
Therefore, 2.40 ×10²⁴ molecules are there in 4.00 moles of glucose, C₆H₁₂O₆.
To know more about molecule, here:
https://brainly.com/question/29254782
#SPJ1
________ iron is found in some foods that provide all the amino acids humans require for the absorption of iron.
Answers
Non-heme iron is found in some foods that provide all the amino acids humans require for the absorption of iron.
What are amino acids?Amino acids are defined as the substances which are considered to be the monomers of proteins.Every amino acid has the same structure which consists of a central carbon bonded to an amino group , carboxyl group and a hydrogen.
Each amino acid also has an another atom or a group of atoms bonded to the alpha carbon which are also known as the R group or the variable group of the side chain.There are 20 common amino acids which are present in natural proteins and each amino acid has the same backbone.
The sequence and number of amino acids determines protein's shape,size and also its function. Each amino acid is attached to the other by a covalent bond formed by a dehydration reaction.
Learn more about amino acids,here:
https://brainly.com/question/28409615
#SPJ6
If you warm up a sealed container of liquid will the numbers of particles inside the sealed container increase decrease or stay the same
Answers
Answer:
stay the same , but the heat will cause the particles to move faster
Explanation:
hope that helps , have a good day :) !!
Find the formula mass of the compound, then divide the individual element total by the total mass-move the decimal over two to change it to percentage SiF4
Answers
Answer:
Explanation:
Here, we start by calculating the formula mass of the compound
We can get this by using the atomic masses of the elements
The atomic mass of silicon is 28 amu
The atomic mass of fluorine is 19 amu
The formula mass of the given compound is thus:
\(28\text{ + 4\lparen19\rparen = 104 g/mol}\)Now, we divide the individual total mass by element by the formula mass
For Silicon, we have:
\(\frac{28}{104}\text{ = 0.27}\)For Fluorine, we have:
\(\frac{76}{104}\text{ = 0.73}\)To fill the valence shell, an electrically neutral, unbonded atom with atomic number 8 must add.
Answers
Answer:
2 electrons
Explanation:
Find the entropy of 1 mole of N2 molecule consider it as an ideal gas occupying a cubic volume of side 1 cm
choices
1: 74 J/K
2: 37
3:136
4: 62
Answers
The entropy of 1 mole of N2 molecules, considered as an ideal gas occupying a cubic volume of side 1 cm, is approximately 4: 62 J/K.
To find the entropy of 1 mole of N2 molecules, we need to use the formula for entropy:
S = R * ln(W)
Where:
S is the entropy
R is the gas constant
ln is the natural logarithm
W is the number of microstates or ways the system can be arranged
For an ideal gas, the number of microstates can be calculated using the formula:
W = (V^N) / (N!)
Where:
V is the volume
N is the number of molecules
Given that we have 1 mole of N2 molecules, which is approximately 6.022 x 10^23 molecules, and the volume of the cube is 1 cm^3, we can calculate the entropy.
First, let's convert the volume from cm^3 to m^3:
1 cm^3 = (1 x 10^-2 m)^3 = 1 x 10^-6 m^3
Now, we can substitute the values into the formula for W:
W = ((1 x 10^-6 m^3)^(6.022 x 10^23)) / (6.022 x 10^23)!
To simplify the calculation, we can use the fact that ln(W) is equivalent to ln((V^N) / (N!)) = ln(V^N) - ln(N!).
ln(W) = ln((1 x 10^-6 m^3)^(6.022 x 10^23)) - ln(6.022 x 10^23)!
ln(W) = (6.022 x 10^23) * ln(1 x 10^-6 m^3) - ln(6.022 x 10^23)!
Now, substituting the values of ln(1 x 10^-6 m^3) and ln(6.022 x 10^23) into the equation:
ln(W) = (6.022 x 10^23) * (-13.8155) - ln(6.022 x 10^23)!
Finally, we can use the value of the gas constant, R, which is approximately 8.314 J/(mol·K), to calculate the entropy:
S = R * ln(W) = (8.314 J/(mol·K)) * ((6.022 x 10^23) * (-13.8155) - ln(6.022 x 10^23)!)
learn more about entropy
https://brainly.com/question/6364271
#SPJ11