6)
Use VSEPR to predict the electron-pair geometry of O2.
A)
linear
B)
trigonal planar
C)
tetrahedral
ientific
Calc
D)
trigonal bipyramidal
Answers
Answer:
A.) Linear
Explanation:
The geometry of the molecular oxygen (O₂) is linear. Thus, option (A) is correct.
What is VSEPR theory?Valence shell electron-pair repulsion theory (VSEPR theory) is used to predict the molecular structure, and bond angles around a central atom of a molecule by using the number of bonds and lone electron pairs in Lewis structure.
The VSEPR model considers that electron pairs present in the valence shell of a central atom have an arrangement so that repulsions between these electron pairs will be minimized by maximizing the distance between them.
The valence electrons of a central atom form either bonding pairs or lone pairs. The electrostatic repulsion of these electrons is minimized when several regions of high electron density maximum possible distance.
The oxygen molecule has linear geometry with a bond angle of 180°. Each oxygen in oxygen molecule has two lone pairs of electrons on it.
Learn more about VSEPR theory, here:
https://brainly.com/question/17177984
#SPJ2
Related Questions
What process results in a new substance being formed?
Chemical reaction
Physical change
Mixing
Answers
Answer:
Chemical reaction
Explanation:
A container has the dimensions of 10cm x 80mm x 0.15m. The density of
its contents is 2.1 g/cm3. What is the mass of the substance in kilograms?
Answers
Answer:
2.52kg
Explanation:
80mm = 8 cm
0.15m = 15 cm
volume of the container = 10m × 8cm × 15cm
= 1200 cubic centimetres
mass = density × volume
= 2.1 × 1200
= 2520g
2520/ 1000
= 2.52kilograms
Calculate the standard reaction enthalpy for the reaction below:
3Fe2O3(s) → 2Fe3O4(s) + ½O2(g)
Answers
The standard reaction enthalpy for the given reaction is +235.8 kJ/mol.
What is the standard reaction enthalpy of reaction?The standard reaction enthalpy (ΔH°) for the given reaction is determined as follows:
Equation of reaction: 3 Fe₂O₃ (s) → 2 Fe₃O₄ (s) + ½ O₂ (g)
The standard enthalpy of formation values for Fe₂O₃ (s), Fe₃O₄(s), and O₂(g) is used to calculate the standard reaction enthalpy.
ΔH° = [2 × ΔH°f(Fe₂O₃)] + [½ × ΔH°f(O₂)] - [3 × ΔH°f(Fe₃O₄)]
where;
ΔH°f(Fe₂O₃) = -824.2 kJ/mol
ΔH°f(Fe₃O₄) = -1118.4 kJ/mol
ΔH°f(O₂) = 0 kJ/mol
ΔH° = [2 × (-1118.4 kJ/mol)] + [½ × 0 kJ/mol] - [3 × (-824.2 kJ/mol)]
ΔH° = -2236.8 kJ/mol + 0 kJ/mol + 2472.6 kJ/mol
ΔH° = 235.8 kJ/mol
Learn more about standard reaction enthalpy at: https://brainly.com/question/15174388
#SPJ1
what is ∆g° for the reaction ch₃oh(g) → co(g) 2 h₂(g) at 25°c?AH° = +90.7 kJ/mol AS° = +221 J/molók kJ/mol 1 2 3 Х 4 5 6 C 7 8 9 O +/- x 100
Answers
The standard Gibbs free energy change (∆G°) for the reaction at 25°C is approximately +24.842 kJ/mol.
The standard Gibbs free energy change (∆G°) for the reaction
CH₃OH(g) → CO(g) + 2 H₂(g) at 25°C
can be calculated using the equation ∆G° = ∆H° - T∆S°.
∆H° = +90.7 kJ/mol and ∆S° = +221 J/(mol·K), we need to convert the units of ∆S° to kJ/(mol·K):
∆S° = +221 J/(mol·K) = +0.221 kJ/(mol·K).
Substituting the values into the equation:
∆G° = ∆H° - T∆S°,
∆G° = +90.7 kJ/mol - (298 K) × (+0.221 kJ/(mol·K)).
Calculating this:
∆G° = +90.7 kJ/mol - 65.858 kJ/mol,
∆G° = +24.842 kJ/mol.
Learn more about energy change here:
https://brainly.com/question/31383716
#SPJ11
How many grams are in 7.23 x 10^24 Al atoms?
Answers
Answer:
323.76 g
Explanation:
The number of grams of the given atoms of Aluminum is 323.73 grams.
The given parameters:
Atom of the Aluminum, = 7.23 x 10²⁴The number of grams of the given atoms of Aluminum is calculated as follows;
6.02 x 10²³ atoms of Aluminum = 27 grams7.23 x 10²⁴ atoms of Aluminum = ?\(= \frac{7.23 \times 10^{24} \times 27 \ g}{6.02 \times 10^{23}} \\\\= 323.73 \ g\)
Thus, the number of grams of the given atoms of Aluminum is 323.73 grams.
Learn more about atoms of element here: https://brainly.com/question/6258301
Why is calcium in group 2A if the modern periodic table
Answers
5
Select the correct answer.
If a stone dropped into a well reaches the water's surface after 3.0 seconds, how far did the stone drop before hitting the water?
ОА
1.4 meters
OB.
44 meters
Ос.
49 meters
OD
54.2 meters
Reset
Next
on samantium All riachte nennund
mentum com/assessments delivery
Answers
Answer:
s= 0.5 at^2
0.5 x 9.81 x 3^2 = 44m
Explanation:
OB.
44 meters
What does “escape velocity” allow a rocket to do?
Answers
Answer:
With escape velocity in a direction pointing away from the ground of a massive body, the object will move away from the body, slowing forever and approaching, but never reaching, zero speed. Once escape velocity is achieved, no further impulse need be applied for it to continue in its escape.
Explanation:
Make sure to edit so you're not copy write
Answer: "With escape velocity in a direction pointing away from the ground of a massive body, the object will move away from the body, slowing forever and approaching, but never reaching, zero speed. Once escape velocity is achieved, no further impulse need be applied for it to continue in its escape".
Explanation: This might help lol
What volume in ml of 0.3000 m nacl solution is required to produce 0.1650 moles of nacl?
Answers
One mole of a substance is defined as the amount of that substance that contains the same number of particles as there are atoms in exactly 12 grams of carbon-12. Approximately 550 mL of the 0.3000 M NaCl solution is required to produce 0.1650 moles of NaCl.
In chemistry, a mole (mol) is a unit of measurement used to quantify the amount of a substance. It is a fundamental concept in stoichiometry and plays a central role in understanding the relationships between the masses, numbers of particles, and volumes of substances involved in chemical reactions.
To determine the volume of a 0.3000 M NaCl solution needed to produce 0.1650 moles of NaCl, we can use the equation:
moles = molarity x volume
Rearranging the equation to solve for volume:
volume = moles/molarity
Given that the moles of NaCl is 0.1650 and the molarity is 0.3000 M, we can substitute these values into the equation:
\(volume = 0.1650 moles / 0.3000 M\\ = 0.55 L\)
To convert the volume from liters to milliliters, we multiply by 1000:
\(volume = 0.55 L * 1000 mL/L\\volume = 550 mL\)
Therefore, approximately 550 mL of the 0.3000 M NaCl solution is required to produce 0.1650 moles of NaCl.
For more details regarding mole, visit:
https://brainly.com/question/30892840
#SPJ4
Which type of stress force produces reverse faults?
a. shearing
b. tension
c. compression
d. deformation
Answers
Answer:
C. Compression
Explanation:
edge 2021
Compression stress force produces reverse faults. Therefore, option (C) is correct.
What are reverse faults?A fault can be described as the rupture of the earth's crust, horizontally, and a reverse fault defines as a 'dip-slip' fault moving vertically. The crust of the earth moves along faults, which are everywhere, both on land as well as on the crust under the oceans.
A reverse fault can be described as a type of dip-slip fault. These faults move vertically and the earth moves up or down relative to these faults. are different kinds of faults, classified by how the earth on either side of the fault moves.
In a normal fault, one side of the fault slides down so when one side of the fault does go up instead of down, it is known as a reverse fault. A special kind of reverse fault is known as a thrust fault.
Therefore, the compression stress force produces reverse faults while the tensional stress force produces normal faults.
Learn more about reverse faults, here:
https://brainly.com/question/16906279
#SPJ2
CAN YOU PLEASE ANSWER
Which observations describe both images? Select all the correct answers. The oil mixes with water. The oil floats on the surface of the water. The oil sinks to the bottom of the water. The oil spreads to cover the water. The oil stays in one spot.
Answers
Answer:
the oil spreads to cover the water
Answer:
I think it is ; The oil floats to the surface of the water
Explanation:
I don't know about the other one.
you are using food labels as a tool to help you avoid purchasing products with trans fatty acids. which of the following would you avoid, since it is the most likely to contain trans fatty acids?
Answers
Among the given options, the most likely to contain trans fatty acids would be partially hydrogenated vegetable oil.
Trans fatty acids are commonly found in processed foods that use partially hydrogenated oils as an ingredient. Partial hydrogenation is a process that converts liquid vegetable oils into solid or semi-solid fats, thereby increasing their shelf life and stability.
However, this process also generates trans fatty acids as a byproduct. Trans fats have been linked to various health issues, including heart disease. To make healthier choices, it is advisable to avoid products that list partially hydrogenated vegetable oil on their food labels.
For more information on fatty acids visit: brainly.com/question/28835959
#SPJ11
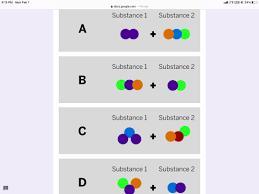
Starting substances
Substance 1
?
Substance 2
+ ?
Jamie works at a company that makes cleaning chemicals. She is trying to make a chemical that smells like flowers. She took two
samples that were gases at room temperature and mixed them in a sealed container.
The diagram above shows the repeating groups of atoms that make up the two starting substances.
After mixing, Jamie found two substances that smelled like flowers in the sealed container. (Nothing had escaped.)
Which of the diagrams shows the repeating groups of atoms that make up the ending substances?
Answers
The combination of the compounds can be found in option C
How is a compound formed?
A compound is formed through a chemical reaction or a combination of elements. In a chemical reaction, two or more elements combine or react with each other to form a compound.
The elements involved in the reaction undergo a rearrangement of their atoms and bonding to form new chemical bonds, resulting in the formation of a compound with different properties from the original elements. This is clear from the images that have been shown in the question.
Learn more about compound:https://brainly.com/question/14117795
#SPJ1

9.
Look at the following equation. What is the oxidizing agent? 3Mg + N2 ->Mg3N2
Answers
Answer:
In this reaction, nitrogen acts as the oxidizing agent. Mg is a reducing agent.
Inside Earth, it is very hot and this source of heat called
Answers
Answer:
Geothermal energy
Explanation:
Hope that helps !!!
Someone please help me out!!!

Answers
The correct options based on the information will be:
b) hydrogen bondsd) HFcovalent bonds (1), hydrogen bonds (2), dipole-dipole forces (3), London dispersion forcesHow to explain the informationEnhanced boiling points can be attributed to heightened intermolecular forces since more energy is demanded to break the bonds between molecules in a liquid state and transform them into a gaseous phase.
The presence of strong intermolecular force characterizes Bromine. Fluorine, on the other hand, serves as a gas when under standard temperature and pressure conditions because it experiences weak intermolecular forces consequent to its nonpolar nature and limited size.
Due to the involvement of polarizable electrons and larger proportions, Bromine remains in its liquid state while kept at standard temperature and pressure, attesting thereby to a stronger intermolecular bond compared with Fluorine's relatively weaker bonding property.
Learn more about bond on
https://brainly.com/question/25965295
#SPJ1
estimate the theoretical chemical oxygen demand for a 100 mg/l solution of methanol (ch3oh).
Answers
Theoretically, a 100 mg/L solution of methanol would have a COD of 2,500 mg/L.
What is oxygen equivalent ?The quantity of oxygen needed to oxidize organic molecules in water is measured by the chemical oxygen demand. A powerful oxidizing agent, such as potassium dichromate (K2Cr2O7), can oxidize methanol (CH3OH), a straightforward organic molecule, when it is present with sulfuric acid (H2SO4).
The balanced chemical equation for the oxidation of methanol by potassium dichromate is:
CH3OH + 2[O] → CO2 + 2H2O
where [O] represents the oxidizing agent.
The following equation can be used to get the theoretical COD for methanol:
COD = (8 × W × 1000) / (32 × V)
where:
W = mass of methanol in the sample (in mg)
V = volume of the sample (in mL)
Substituting the values given:
W = 100 mg (since the solution concentration is 100 mg/L)
V = 1000 mL (assuming a 1 L sample)
COD = (8 × 100 mg × 1000) / (32 × 1000 mL) = 2,500 mg/L
Therefore, Theoretically, a 100 mg/L solution of methanol would have a COD of 2,500 mg/L.
Learn more about oxygen equivalent here : brainly.com/question/28788332
#SPJ4
two atoms With diferent mass number but the same atomic number are called
Answers
Answer:
they are called isotopes
When NH4SCN is added to a solution of Fe3+ ions, the solution changes from yellow to orange. What is the best explanation for this color change?
Answers
Answer:
The iron present in the solution is reduced because of the presence of SCN⁻ ions in solution
Explanation:
The clue in this problem is the color of the solution:
A solution of Fe³⁻ is yellow but when the Fe³⁺ is reduced by an oxidant to Fe²⁺ the solution change to orange color.
The SCN⁻ ion is a reducing agent than, in presence of a substance that can be reducted as Fe³⁺ ion will promote the reaction. That means the best explanation is:
The iron present in the solution is reduced because of the presence of SCN⁻ ions in solutionI need help matching these
Gravitational energy
Mechanical energy
Nuclear energy
Thermal energy
A) Energy stored in the nucleus of an atom
B) Internal energy caused by vibrations of atoms and molecules
C) The energy of an object due to its movement
D) The potential energy of position
Answers
The correct matching of the various energy forms are as follows:
Nuclear energy- Energy stored in the nucleus of an atomThermal energy - Internal energy caused by vibrations of atoms and moleculesMechanical energy - the energy of an object due to its movementGravitational energy - the potential energy of positionWhat is energy?Energy is defined as the ability to do work.
There are various forms of energy such as:
Gravitational energyMechanical energyNuclear energyThermal energyThe correct matching of the various energy forms are as follows:
Nuclear energy- Energy stored in the nucleus of an atomThermal energy - Internal energy caused by vibrations of atoms and moleculesMechanical energy - the energy of an object due to its movementGravitational energy - the potential energy of positionThe various forms of energy can be interconverted from one form to another.
In conclusion, energy is the ability to do work and it exists in various forms which can be interconverted.
Learn more about energy at: https://brainly.com/question/13881533
#SPJ1
In your own words, explain how a compound could experience a chemical change.
Answers
Answer:
a compound can experience a chemical change if foreign body are in troduced
What is the distance traversed by the particle between 0 seconds and 6 seconds
Answers
The distance travelled by the particle between 0 seconds and 6 seconds is 12 m
What is velocity?Velocity is simply defined as the rate of change of displacement with time. Mathematically, it can be expressed as:
Velocity = displacement / time
From the question given above, the following data were obtained:
Time = 6 sVelocity = 2 m/sDisplacement =?Velocity = displacement / time
The displacement of the object between 0 and 6 s is calculated as;
2 = displacement / 6
Cross multiply
Displacement = 2 × 6
Displacement = 12 m
Learn more about velocity here:
brainly.com/question/3411682
#SPJ1

can use greenhouse gases or wasted heat or by-products as input for process to generate any form of energy (in simple way in small scale as a prototype)
Answers
2 agNO3+cu>cu(NO3)2 +2 AG What is evidence that a chemical reaction took place
Answers
The evidence that a chemical reaction took place in 2 AgNO3 + Cu → Cu(NO3)2 + 2 Ag includes colour change, precipitation, the redox process, and the formation of new substances. These changes all point towards a chemical reaction that has occurred.
In the given chemical reaction, 2 AgNO3 + Cu → Cu(NO3)2 + 2 Ag, there are several pieces of evidence that indicate a chemical reaction took place:
1. Color change: As the reaction progresses, you'll notice a change in colour. The initial colour of copper (Cu) is reddish-brown, while silver nitrate (AgNO3) is colourless. After the reaction, the colourless silver (Ag) and blue-green copper nitrate (Cu(NO3)2) are formed.
2. Precipitation: During the reaction, solid silver (Ag) precipitates out of the solution. This change from a soluble to an insoluble substance indicates a chemical reaction.3. Redox process: The reaction involves an exchange of electrons between the elements, as copper loses two electrons (oxidation) and each silver ion gains one electron (reduction). This redox process is a sign of a chemical reaction taking place.
4. Formation of a new substance: The reaction produces two new substances, silver (Ag) and copper nitrate (Cu(NO3)2), which did not exist before the reaction. The creation of new substances is a strong indication that a chemical reaction occurred.
To learn more about silver nitrate, refer:-
https://brainly.com/question/29627918
#SPJ11
Assume you need to achieve a nitrogen concentration of 0.52 wt% at a position 5 mm into an iron-nitrogen alloy that initially contains 0.08 wt% N. The surface concentration is to be maintained at 1.00 wt% N, and the treatment is to be conducted at 1,100 K. (D. = 9.10E-05 m2/s and Qd = 168 kJ/mol) 25) Find the diffusion coefficient at 1,100 K if k=8.31 a) 8.91x10-12 m2/s b) 9.49x10-13 m²/s c) 7.44x10-11 m2/s d) 4.39x10-12 m2/s e) NoA
Answers
The diffusion coefficient is 4.39x10-12 m2/s.
Given information;
Initial nitrogen concentration, c₀ = 0.08 wt %
Nitrogen concentration to be achieved, cₙ = 0.52 wt %
Diffusion coefficient, D = 9.10E-05 m²/s
Temperature, T = 1100 K
Activation energy, Qd = 168 kJ/mol
Gas constant, R = 8.31 J/mol K
To find;
Diffusion coefficient at 1100 K using Arrhenius equation;
The Arrhenius equation for diffusion coefficient is given as;
D = D₀ exp(-Qd / R T)
where; D₀ is the diffusion coefficient at an infinite temperature.
Substituting the given values of D, Qd, R, and T into the equation above;
D = 9.10E-05 m²/s
Qd = 168 kJ/mol
R = 8.31 J/mol
KT = 1100 K
At 1100 K, the value of kT is;
kT = R T
= 8.31 J/mol K x 1100 K
= 9141 J/mol
Multiplying by Avogadro's number to get the value in J;
9141 J/mol x (6.022 x 10²³) / (1 mol) = 5.50 x 10²⁹ J-1
= 5.50 x 10²⁹ m²/kg
Multiplying by the Boltzmann constant to get the value in m²/s;
K = 1.38 x 10⁻²³ J/KD₀ can now be obtained by rearranging the Arrhenius equation as;
D₀ = D / exp(-Qd / R T)
Substituting the values into the equation;
D₀ = 9.10E-05 m²/s / exp(-168 x 10³ J/mol / 8.31 J/mol K x 1100 K)D₀
= 9.10E-05 m²/s / exp(-21.36)D₀
= 9.10E-05 m²/s / 1.29E-09D₀
= 7.05E-04 m²/s
Therefore, the diffusion coefficient at 1,100 K if k = 8.31 is;
D = D₀ exp(-Qd / R T)D
= 7.05E-04 m²/s exp(-Qd / R T)D
= 7.05E-04 m²/s exp(-168 x 10³ J/mol / 8.31 J/mol K x 1100 K)
D = 7.05E-04 m²/s exp(-21.36)D
= 4.39 x 10⁻¹² m²/s
Therefore, the correct option is 4.39x10-12 m2/s.
Learn more about Concentration from the given link :
https://brainly.com/question/17206790
#SPJ11
In the compound AbO3, the ratio of aluminum to
oxygen is
A) 3 moles of aluminum to 2 moles of oxygen
B) 2 moles of aluminum to 3 moles of oxygen
C) 2 grams of aluminum to 3 grams of oxygen
D) 3 grams of aluminum to 2 grams of oxygen
Answers
Answer:
c
Explanation:i got it right ony my test
Of the two bromoderivatives, C6H5CH(CH3)Br and C6H5CH(C6H5)Br which one is more reactive in SN1 substitution reaction and why?
Answers
Answer:
tertiary
Explanation:
tertiary halogenoalkanes are more reactive than primary and secondary as the carbocation is more stable due to alkyl groups( have high electron density) donating electrons to stabilise the carbocation
1. The elements at the bottom of the table were pulled out to keep the table from
becoming too long. The first period at the bottom called the
Answers
Answer: Lanthanoids
Explanation:
What effect would the chemical in a bee sting have on litmus paper?
Answers
Answer:
A bee sting has a ph of 5, and is therefore an acid. Litmus paper goes red (or stays red) in acidic solutions.
Answer:
The Blue Litmus Paper should turn red
Explanation:
Bee venom has a pH of 5.5, which means it is mildly acidic which in turn should make the Litmus paper turn Red. Hope this helps!
HELP ILL GIVE BRAINLIEST
An epicenter station that records seismic waves is 7,000 km
away from the earthquake epicenter. How long after the first
P-wave did the first S-wave arrive?

Answers
Answer:
8.7min
Explanation:
The epicenter is the origin of an earthquake wave.
Since the distance between the earthquake origin and the station is 7000km and we are told to find the time after which the s - wave arrives from that of the p- waves, we follow the procedure below:
we use the graph of the travel time - distance giventrace the 7000km as the distance and find where it cuts the p-wave curve. the point is 10.3min trace it up to where 7000km intersects the s-wave curve which is at 19minSo;
The time difference = 19min - 10.3min = 8.7min
After the p-waves arrived, it took the s-waves 8.7min to reach the station.
Answer:
8.7min
Explanation:
The epicenter is the origin of an earthquake wave.
Since the distance between the earthquake origin and the station is 7000km and we are told to find the time after which the s - wave arrives from that of the p- waves, we follow the procedure below:
we use the graph of the travel time - distance given
trace the 7000km as the distance and find where it cuts the p-wave curve.
the point is 10.3min
trace it up to where 7000km intersects the s-wave curve which is at 19min
So;
The time difference = 19min - 10.3min = 8.7min
After the p-waves arrived, it took the s-waves 8.7min to reach the station.
plz mark me as brainliest.