Answers
Answer:
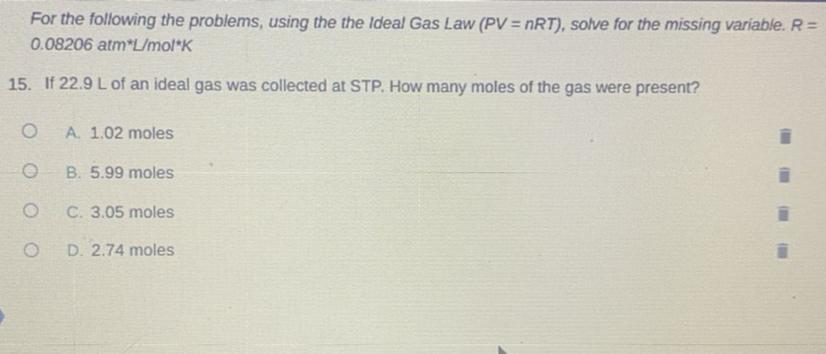
A. 1.02 moles .
Explanation:
Hello!
In this case, given the ideal gas equation, as we need to solve for moles, we divide both sides by RT to get:
\(n=\frac{PV}{RT}\)
Thus, by plugging in the pressure and temperature at STP (1.00 atm and 273.15 K respectively) we obtain:
\(n=\frac{1.00atm*22.9L}{0.08206\frac{atm*L}{mol*K}*273.15K}\\\\n=1.02mol\)
Therefore, the correct answer is A. 1.02 moles
Best regards!
Related Questions
please help me please help me

Answers
Description: When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). This reaction is called the Fischer esterification. Notes: The reaction is actually an equilibrium. The alcohol is generally used as solvent so is present in large excess.
How is energy related to the change of state represented by the model?
Atoms gain energy as a solid changes to a liquid.
Atoms gain energy as a solid changes to a gas.
Atoms lose energy as a solid changes to a liquid.
Atoms lose energy as a solid changes to a gas.
Answers
Answer:
Atoms gain energy as a solid changes to a liquid. If atoms energy during a change of state, they are pulled together by attractive forces and become more organized.
Answer:
its B
Explanation:
A.) The flask on the right is the strong acid because only some of the molecules dissociate.B.) The flask on the left is the strong acid because 100% of the molecules dissociate.C.) The flask on the right is the strong acid because 100% of the molecules dissociate.D.) The flask on the left is the strong acid because only some of the molecules dissociate.

Answers
Answer:
B.) The flask on the left is the strong acid because 100% of the molecules dissociate.
Explanation:
First, let's review the concept of strong acid: A strong acid is an acid that is completely ionized in an aqueous solution. Let's see an example of this:
\(HCl\rightarrow H^++Cl^-.\)Now, you can see the picture and imagine that the grey circles are Cl and the black circles are H, so as HCl is a strong acid and it dissociates completely in two different ions, the strong acid would be the first flask. It wouldn't be the second flask because there are some molecules that don't dissociate completely (because they are not 'separated').
Based on this logic, the answer would be B.) The flask on the left is the strong acid because 100% of the molecules dissociate.
Mg(NO3)2 soluble or insoluble?
Answers
Answer:
The chemical compound Mg(NO3)2, also known as magnesium nitrate, is very soluble, especially in water.
What is the ΔG (kJ/mol) for a reaction at 25 Celsius that is:
Mg3(PO4)2 (s) ⇄ 3 Mg2+ (aq) + 2 PO43− (aq) ΔG0 = 137.0 kJ/mol
If there is initially 0.65 M Mg2+(aq) and 0.43 M PO43− (aq) in solution?
Answers
Answer:
115.6 kJ/mol
Explanation:
The ΔG of a reaction can be calculated using the following equation:
ΔG = ΔG° + RT ln(Q)
where:
ΔG° is the standard free energy change, which is given as 137.0 kJ/mol in this case
R is the gas constant, which is 8.314 J/(mol·K)
T is the temperature in Kelvin, which is 25°C + 273.15 = 298.15 K
Q is the reaction quotient, which is the ratio of the concentrations of the products to the concentrations of the reactants, each raised to their stoichiometric coefficients.
From the chemical equation given, the stoichiometric coefficients of Mg2+ and PO43- are 3 and 2 respectively. Therefore, the reaction quotient can be expressed as:
Q = [Mg2+]^3 [PO43-]^2
Substituting the given initial concentrations of Mg2+ and PO43- into the reaction quotient expression, we get:
Q = (0.65 M)^3 (0.43 M)^2 = 0.011 M^5
Now we can calculate the ΔG of the reaction:
ΔG = ΔG° + RT ln(Q)
ΔG = (137.0 kJ/mol) + (8.314 J/(mol·K) × 298.15 K) × ln(0.011 M^5)
ΔG = 137.0 kJ/mol - 21.38 kJ/mol
ΔG = 115.6 kJ/mol
Therefore, the ΔG for the reaction at 25°C and the given initial concentrations of Mg2+ and PO43- is 115.6 kJ/mol.
Rank the following iron-carbon alloys and associated microstructures from the hardest to the softest: __________.
(a) 0.25 wt% C with coarse pearlite,
(b) 0.80 wt% C with spheroidite,
(c) 0 25 wt% C with spheroidite, and
(d) 0.80 wt% C with fine pearlite.
Justify this ranking.
Answers
Answer:
The Ranking from
Hardest to softest is as follows :
0.80 wt% C with fine pearlite.
0.80 wt% C with spheroidite
0.25 wt% C with coarse pearlite
0.25 wt% C with spheroidite
Explanation:
To find - Rank the following iron-carbon alloys and associated microstructures from the hardest to the softest. Justify this ranking.
Solution -
Ranking is as follows :
(d) 0.80 wt% C with fine pearlite.
(b) 0.80 wt% C with spheroidite
(a) 0.25 wt% C with coarse pearlite
(c) 0 25 wt% C with spheroidite
Justification -
For some wt% C,
Fine pearlite is stronger than spheroidite
and
Coarse pearlite is stronger than spheroidite.
Now,
Due to carbon content,
0.80 wt% C with spheroidite is stronger than 0.25 wt% C with coarse pearlite.
So,
The Ranking from
Hardest to softest is as follows :
0.80 wt% C with fine pearlite.
0.80 wt% C with spheroidite
0.25 wt% C with coarse pearlite
0.25 wt% C with spheroidite
Draw the curved arrow mechanism for the addition of HCN in water with NaOH to 3,4-dimethylcyclopentan-1-one to give the corresponding cyanohydrin in the fewest steps. Draw the arrows that lead to the resonance structures with full octets around each atom other than hydrogen. Draw all electrons and charges if necessary on all structures; do not show any inorganic side products or counterions. Reagents needed for each step are provided in the boxes.
Answers
Answer:
See picture and explanation below
Explanation:
In the attached picture you have the answer for this.
In the first step, we have an acid base reaction between HCN and NaOH. When this happens, its formed the following:
HCN + NaOH --------> CN⁻ + H₂O + Na⁺
As a second step, The CN⁻ attacks the carbonile group in the cyclopentane. This causes to open the double bond, and then, the Cyano enters the molecule.
The final step is another acid base reaction, where the oxygen substracts a hydrogen atom of the water in the medium, and then, the alcohol group is formed. With this step, the cyanohydrin is finally formed. See the mechanism below in the picture.
Hope this helps

Which of the following are products of the reaction listed? Zn + 2HCI
ZnCl2 + H2
H2
НСІ
ZnCl2
Zn
Answers
Answer:
ZnCl2
H2
Explanation:
The products of the given reaction is:
ZnCl₂ and H₂
The two species are the products of the chemical combination
This reaction is a single displacement reactionBecause Zn is more reactive, it displaces hydrogen from the acidThis forms a hydrogen gasWe know this because Zn is higher in the activity series.HELP ASAP PLEASE ANSWER BOTH OF THESE QUESTIONS! I WILL GIVE YOU BRAINELIST IF YOU ANSWER IT!


Answers
Answer:
because it absorbs all colours expect for red,
Explanation: making the red light bounce into your eyes, therefore seeing the colour red
Question 2: The wave transfers its energy to the mineral, I think, thats my best guess
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon, hydrogen, and oxygen) produced 39.61 g CO2 and 9.01 g H2O. The molar mass of equilin is 268.34 g/mol. Find its molecular formula.
Answers
Answer: The molecular formula for the given organic compound is \(C_{18}H_{20}O_2\)
Explanation:
The chemical equation for the combustion of hydrocarbon having carbon, hydrogen and oxygen follows:
\(C_xH_yO_z+O_2\rightarrow CO_2+H_2O\)
where, 'x', 'y' and 'z' are the subscripts of Carbon, hydrogen and oxygen respectively.
We are given:
Mass of \(CO_2=39.61g\)
Mass of \(H_2O=9.01g\)
We know that:
Molar mass of carbon dioxide = 44 g/mol
Molar mass of water = 18 g/mol
For calculating the mass of carbon:
In 44 g of carbon dioxide, 12 g of carbon is contained.
So, in 39.61 g of carbon dioxide, \(\frac{12}{44}\times 39.61=10.80g\) of carbon will be contained.
For calculating the mass of hydrogen:
In 18 g of water, 2 g of hydrogen is contained.
So, in 9.01 g of water, \(\frac{2}{18}\times 9.01=1.00g\) of hydrogen will be contained.
Mass of oxygen in the compound = (13.42) - (10.80 + 1.00) = 1.62 g
To formulate the empirical formula, we need to follow some steps:
Step 1: Converting the given masses into moles.Moles of Carbon = \(\frac{\text{Given mass of Carbon}}{\text{Molar mass of Carbon}}=\frac{10.80g}{12g/mole}=0.9moles\)
Moles of Hydrogen = \(\frac{\text{Given mass of Hydrogen}}{\text{Molar mass of Hydrogen}}=\frac{1g}{1g/mole}=1moles\)
Moles of Oxygen = \(\frac{\text{Given mass of oxygen}}{\text{Molar mass of oxygen}}=\frac{1.62g}{16g/mole}=0.10moles\)
Step 2: Calculating the mole ratio of the given elements.For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 0.10 moles.
For Carbon = \(\frac{0.9}{0.10}=9\)
For Hydrogen = \(\frac{1}{0.10}=10\)
For Oxygen = \(\frac{0.10}{0.10}=1\)
Step 3: Taking the mole ratio as their subscripts.The ratio of C : H : O = 9 : 10 : 1
Hence, the empirical formula for the given compound is \(C_9H_{10}O\)
For determining the molecular formula, we need to determine the valency which is multiplied by each element to get the molecular formula.
The equation used to calculate the valency is :
\(n=\frac{\text{Molecular mass}}{\text{Empirical mass}}\)
We are given:
Mass of molecular formula = 268.34 g/mol
Mass of empirical formula = 134 g/mol
Putting values in above equation, we get:
\(n=\frac{268.34g/mol}{134g/mol}=2\)
Multiplying this valency by the subscript of every element of empirical formula, we get:
\(C_{(9\times 2)}H_{(10\times 2)}O_{(1\times 2)}=C_{18}H_{20}O_2\)
Thus, the molecular formula for the given organic compound is \(C_{18}H_{20}O_2\).
Given the reaction, how many moles of Z will be produced from 4.85 mol A, assuming excess B?
2 A +3B = 4Y + 5 Z
Answers
Answer:
12.125 moles of Z
Explanation:
Hi! Here, since you only know the number of moles for one of the reactants, you will use the coefficients in the reaction to use the mole ratio to find your answer. Since the question states that there are 4.85 mol A and excess B, A is your limiting reactant. This means that once all of the A is used up in the reaction, you will have your maximum amount of Z left behind because there will only be B left and no A to make more Z.
The mole ratio of A:Z is 2:5, found from the coefficients in the chemical equation(2 moles of A for every 5 moles of Z).
So, start with the information that is given to you (4.85 mol A) and then use the mole ratio, like this:
4.85 mol A(\(\frac{5 mol Z}{2 mol A}\))
This way, the mol A will cancel out, leaving behind mol Z (always put the unit you are looking for on top).
Doing this leads to mol Z = 12.125 mol.
I hope this helps! Good luck with your finals!
As per the given 4.85 moles of A will produce 12.125 moles of Z, assuming excess B.
What is chemical reaction?A chemical reaction is the transformation of one or more substances, known as reactants, into one or more different substances, known as products.
Chemical reactions involve the breaking and forming of chemical bonds between atoms and molecules, causing atoms to rearrange and the chemical and physical properties of the substances involved to change.
From the balanced chemical equation, we can see that 2 moles of A react with 3 moles of B to produce 5 moles of Z.
So the mole ratio of A to Z is 2:5.
Therefore, if 2 moles of A react to produce 5 moles of Z, then 4.85 moles of A will produce:
(4.85 mol A) x (5 mol Z / 2 mol A) = 12.125 mol Z
Thus, 4.85 moles of A will produce 12.125 moles of Z, assuming excess B.
For more details regarding chemical reaction, visit:
https://brainly.com/question/29762834
#SPJ2
A chemist weighed out 8.72 g of tin. Calculate the number of moles of tin she weighed out.
Be sure your answer has the correct number of significant digits.
Answers
-to go from grams to moles we multiply:
grams (mol/grams)
-grams will cancel and we will be left with moles.
-Plug in your values; the atomic mass of tin (Sb) is 118.7g. (one mol of tin is equal to 118.7g):
8.72g (1 mol/118.7g) = 0.07346251
-since 1 mole of tin is equal to 118.7g, this is just like multiplying the initial value by 1; we are, in fact, not changing the number. Just converting to another unit of measure.
-round to the least number of significant digits (3 sig fig) and we end with 0.0735 mol.
How much energy is needed to cool a 250g cup of water from 23 °C to -23 °C?



Answers
During the cooling process, we can identify three stages, as shown in the figure attached to the question.
1. Water cooling from 23°C to 0°C, freezing temperature of the water. The energy for this stage will be:
\(Q_1=mCp_1\Delta T\)Where,
m is the mass of water, 250g
Cp1 is the specif heat of water, 4.186J/g°C
dT is the difference of temperature, T2-T1=0°C-23°C=-23°C
\(Q_1=250g\times4.186\frac{J}{g\degree C}\times-23\degree C=-24069.5J\)We have a negative value because the process releases energy
2. Change of phase of water, from liquid water to ice. This process occurs at a constant temperature equal to 0°C. The energy for this stage will be:
\(Q_2=-m\Delta Hf\)dHf is the heat of fusion. We put a negative sign because we have the contrary process of fusion, freezing.
\(\begin{gathered} Q_2=250g\times-334J/g \\ Q_2=-83500J \end{gathered}\)3. Ice cooling from 0°C to -23°C.
\(Q_3=mCp_3\Delta T\)Where,
m is the mass of ice, 250g
Cp3 is the specific heat of ice, 2.1J/g°C
dT is the difference of temperature, -23°C-0°C=-23°C
\(\begin{gathered} Q_3=250g\times2.1\frac{J}{g\degree C}\times-23\degree C \\ Q_3=-12075J \end{gathered}\)So, the total energy released will be:
\(\begin{gathered} Q_T=Q_1+Q_2+Q_3 \\ Q_T=119644.5J=-119.6kJ \end{gathered}\)Answer: The total energy will be -119.6kJ, which means that the energy is released.
Calculate the pH of the following aqueous solution:
0.35 M NaF (pKa for HF = 3.14)
I'm pretty sure I know how to calculate the pH by itself, but I am confused on how to get the equation. I'm not sure what HF has to do with this, other than it's an acid. I'm probably overthinking it because it's worded differently. Thanks in advance!
Answers
The pH of an aqueous solution that has a concentration of 0.35 M NaF and pKa for HF = 3.14 is 3.6.
How to calculate pH?The pH of a solution refers to the degree of acidity or alkalinity of the solution. It can be calculated using the Henderson-Hasselbalch Equation as follows:
pH = pka + log ([A-]/[HA])
Where;
A- = conjugate baseHA = weak acidpH = pKa + log([F-]/[HF])
pH = 3.14 + log(1/0.35)
pH = 3.14 + 0.4559 = 3.595
Therefore, the pH of an aqueous solution that has a concentration of 0.35 M NaF and pKa for HF = 3.14 is 3.6.
Learn more about pH at: https://brainly.com/question/15289741
________ and ________ are combined to create a healthy, nutritious diet.
macronutrients, refined carbohydrates
micronutrients, macronutrients
macronutrients, fiber
micronutrients, fiber
Answers
Macronutrients and micronutrients are combined to create a healthy, nutritious diet.
What is a Healthy diet?
This type of diet comprises of macronutrients and micronutrients in which the former is responsible for provision of energy to the body.
The latter helps to digest the macronutrients thereby making option B the most appropriate choice.
Read more about Healthy diet here https://brainly.com/question/977885
#SPJ1
Answer:
Macronutrients and micronutrients
Explanation:
Rank from most ionic to least ionic
a. WO3
b. MnS
c. MnS2
d. ZnS
e. ZrS2
Answers
The order of increasing ionic property is;
WO3 < ZrS2 < MnS2< ZnS < MnS
Ionic compounds are compounds that contain an ion pair. Typically, ionic compounds are formed between metals and nonmetals.
The degree of ionic character depends on the type of metal involved and the magnitude of charge it carries.
Typically, first row transition metals form ionic compounds. The degree of ionic character depends on the row in which the metal is found and the magnitude of charge it carries.
Hence, the order of ionic character of the compounds from most ionic to least Ionic is; WO3 < ZrS2 < MnS2< ZnS < MnS.
Learn more;https://brainly.com/question/25150590
Consider the following reaction:
CH4(g) + 3Cl₂(g) → CHC13(1) + 3
Balance the equation.
How many grams of Cl₂ are needed to produce 1.25 moles CHC13?1.25mol CHC 13 x 3 mol 1₂ -3.75 mol C1₂
Imol CHCI3
3.75mol Cl₂ x 10.99 265.89 moles per gram
(mol
How many grams of HCl are produced from 0.64 moles CH4?
HCl(g)
How many grams of Cl₂ will produce 11.9 grams of CHC13?

Answers
264.5 grams of Cl₂ are needed to produce 1.25 moles of CHCl₃.; 74.4 grams of HCl are produced from 0.64 moles of CH₄.; 51.2 grams of Cl₂ are needed to produce 11.9 grams of CHCl₃.
How is the equation balanced?To balance the equation, we need to make sure that number of atoms of each element is the same on both the sides of equation.
The balanced equation is: CH₄(g) + 3Cl₂(g) → CHCl₃(l) + 3HCl(g)
1.25 mol CHCl₃ x (3 mol Cl₂/1 mol CHCl₃.) x (70.91 g Cl2/1 mol Cl₂) = 264.5 g Cl₂
Therefore, 264.5 grams of Cl₂ are needed to produce 1.25 moles of CHCl₃.
0.64 mol CH₄ x (3 mol HCl/1 mol CH₄) x (36.46 g HCl/1 mol HCl) = 74.4 g HCl
Therefore, 74.4 grams of HCl are produced from 0.64 moles of CH₄.
11.9 g CHCl₃ x (1 mol CHCl₃/119.38 g CHCl₃) x (3 mol Cl2/1 mol CHCl₃) x (70.91 g Cl₂/1 mol Cl₂) = 51.2 g Cl₂
Therefore, 51.2 grams of Cl₂ are needed to produce 11.9 grams of CHCl₃.
To know more about chemical equation, refer
https://brainly.com/question/23877810
#SPJ1
Calculate the molar mass of ethanol, C2H6O, then use it to convert 6.75 grams of ethanol to moles. Show work
Answers
Using the molecular weight a calculator or the molar mass of CH₃CH₂OH, 1 gram Ethanol = 0.021706834440237 mole.
Is ethanol used to make gasoline?Ethanol is an renewable transportation fuel that is produced in the United States. Ethanol contributes to lower emissions when used in low-level blends like E10 10% ethanol, 90% fuel), E15 10.5% in 15% ethanol as a or E85 flex boost)—a gasoline-ethanol mix containing 51% to 83% a solution of depending on geography or season.
Why is ethanol classified as an alcoholic beverage?Ethanol (CH₃CH₁OH) is a chemical compound (alcohol) that contains the group hydroxyl (OH) bonded to a the carbon atom. Ethanol is made by fermenting crop residue such as sugar cane, maize, and manioc.
To know more about ethanol visit
brainly.com/question/25002448
#SPJ1
How many grams of aluminum oxide will you need to start with in order to make 6500 g of aluminum hydroxide?
Answers
Answer:
Explanation:
2Al(s) + 3 2 O2(g) → Al2O3(s) And given the stoichiometry ...and EXCESS dioxygen gas...we would get 6.25⋅ mol of alumina. the which represents a mass... ...6.25 ⋅ mol ×101.96 ⋅ g ⋅ mol−1 molar mass of alumina ≡ 637.25 ⋅ g.
Answer:
56.7 grams of aluminum oxide (AI²O³) are produced.Need a fast expert's response.
What is the average atomic mass of 10 hydrogen -1 molecules?
Answers
Answer:
1.674 x 10^-23 grams
Explanation:
Hydrogen-1 is called Protium
wikipedia
atomic mass of Protium is 1.00794 amu
sciencedirectcom
atomic mass of 10 Protiums is 10.0794 amu
10.0794 amu in grams is
1.6737236x10^-23 grams
2. Water is an excellent solvent. With water being a polar
molecule, it can be an especially strong solvent for other
polar molecules. Give an example of how water acting as a
solvent is important for living organisms.
NO LINKS PLZ HELP
Answers
Answer:
Water acts as a solvent of carbon dioxide excreted from tissues, in blood and helps in regulating pH (because it forms carbonic acid that lowers pH when it tends to get higher). Blood pH should be maintained at about 7.4. One major reason is that the structure of protein is dependent ton pH because pH determines its ionization hence also affecting the charges and interaction between side groups of amino acids. A change in pH may, therefore, denature proteins and negatively affect cellular functions.
Explanation:
To get more on how water acts as a universal solvent check out brainly.com/question/7007192
3. A tank of compressed CO2 has a pressure of 850 psi and a volume of 150 mL. What is the volume of this gas when the pressure is 45 psi?
Answers
The volume of gas when the pressure is 45 psi is 2,833.33mL.
How to calculate volume?The volume of a substance can be calculated using Boyle's law equation as follows:
P₁V₁ = P₂V₂
Where;
P₁ and V₁ = initial pressure and volumeP₂ and V₂ = final pressure and volumeAccording to this question, a tank of carbondioxide has a pressure of 850 psi and a volume of 150 mL. The volume can be calculated as follows:
850 × 150 = 45 × V
V = 127,500 ÷ 45
V = 2,833.33mL
Therefore, 2,833.33mL is the volume of the gas.
Learn more about volume at: https://brainly.com/question/1437490
#SPJ1
A tumor marker is being developed to detect early breast cancer. Of the 400 women who volunteered for the study, 350 tested negative for the marker. Of these women, three developed breast cancer. The 50 women who tested positive for the marker underwent further tests, of these 50 women, 48 were diagnosed with early breast cancer, and the other two women were found to not have breast cancer. Use enclosed excel sheet for your answers (including your calculations)
Answers
While tumor marker test results can be useful, they are not conclusive. A low result does not imply that you do not have cancer or that you are in remission.
CA 15-3, for example, is raised in less than half of patients with early breast cancer and in more than 80% of those with metastatic breast cancer. Three tumor markers, cancer antigen 15-3 (CA 15-3), cancer antigen 27.29 (CA 27.29), and carcinoembryonic antigen (CEA), have been used in breast cancer care to help monitor metastatic breast cancer (advanced disease), but they have not been found to be useful in detecting a breast cancer recurrence or extending lives. If the level falls, the treatment is effective. If it rises, the cancer may be spreading.
Learn more about effective here-
https://brainly.com/question/27325201
#SPJ4
Is a solution with a low pH more or less acidic than one with a high pH?
Answers
Calculate the mass of 9.96 times 10 to the power of 26 atoms of tellurium
Answers
Answer:
21121.58 grams
Explanation:
Given, 9.96 times 10 to the power of 26 atoms of tellurium
Convert atoms into moles of tellurium,
As we know, 1 mol= 6.02 *10 to the power of 23 atoms
Hence, 9.96 *10 to the power of 26/ 6.02 *10 to the power of 23= 1654 moles of tellurium
Now, mass= molar mass*moles
As we know, tellurium has a molar mass of 127.6000 g/mol
Mass= 1654 *1277 g/mol
= 21121.58
Help please I need help fast.

Answers
Answer:
d) It incorrectly describes the two processes
Hope it helps you
And please mark it as the brainliest answer
Can someone please help me please?

Answers
Answer:
b
Explanation:
Predict and explain the structure of the major and minor products when hydrogen bromide is added to 2-methylbut-2- ene, (Ch3)2CCHCH3
Pls help with homework!!!!
Answers
When hydrogen bromide (HBr) is added to 2-methylbut-2-ene ((CH3)2CCHCH3), an electrophilic addition reaction takes place, where the π bond of the alkene is broken, and the hydrogen and bromine atoms are added to the resulting carbocation.
The reaction proceeds through a Markovnikov addition, where the hydrogen atom attaches to the carbon atom with the greater number of hydrogen atoms.
In this case, the initial addition of HBr to 2-methylbut-2-ene leads to the formation of a primary carbocation, as the positively charged carbon atom only has one alkyl group attached to it. The primary carbocation is relatively unstable, and it can undergo a rearrangement to form a more stable secondary carbocation.
The major product that is typically obtained is the 2-bromo-2-methylbutane. The hydrogen atom from HBr adds to the carbon with three hydrogen atoms (the more substituted carbon), resulting in the formation of a secondary carbocation.
On the other hand, a minor product is also formed, which is 3-bromo-2-methylbutane. This product arises from the addition of HBr to the primary carbocation, which is less stable. Although the primary carbocation is less favored, it can still be formed and lead to the formation of the minor product.
In summary, the addition of HBr to 2-methylbut-2-ene yields two products: the major product is 2-bromo-2-methylbutane, resulting from the addition of HBr to the more stable secondary carbocation, and the minor product is 3-bromo-2-methylbutane, originating from the less stable primary carbocation.
For more such questions on electrophilic addition visit:
https://brainly.com/question/9643304
#SPJ8
Which statements are TRUE about fossil fuels? (Select all that apply.)
They are in a limited supply.
They do not replenish themselves.
They are expensive to extract compared to other forms of energy.
They release large amounts of carbon dioxide when burned
✎help its an exam✎ ☕︎if any links I WILL REPORT☕︎
Answers
Answer:
All is Correct
Explanation:
Fossil fuels have the following properties:
They are in a limited supply. Fossil fuels are non-renewable resources, meaning that they cannot be replenished at the same rate as they are consumed. Once they are used up, they are gone forever.They do not replenish themselves. Fossil fuels take millions of years to form under specific geological conditions. They cannot be regenerated by natural processes in a human timescale.They are expensive to extract compared to other forms of energy. Fossil fuels require complex and costly methods to locate, drill, mine, transport, and refine. They also have negative externalities, such as environmental damage, health risks, and social conflicts, that are not reflected in their market prices.They release large amounts of carbon dioxide when burned. Fossil fuels contain carbon that was stored underground for millions of years. When they are burned, they release carbon dioxide (CO2) into the atmosphere, which is a greenhouse gas that contributes to global warming and climate change.Therefore, the answer is to select all
Answer:
It's A, B, and D
Explanation:
Maybe not D, because that is burning wood like charcoal. Not sure about that. Hope this helps!
Which metal does not react with dilute hydrochloric acid?
A. Iron
B. Sodium
C. Zinc
D. Copper
Answers
Answer:
D. Copper
Explanation:
Less reactive metals like Copper, gold and silver does not react with dilute HCl to produce Hydrogen gas.
Answer:
D. Copper
Explanation:
Copper is less reactive than Hydrogen.