5. Write the chemical formulae of the following chemical compounds.
(i)
Sodium sulphate ..........
(ii) Potassium nitrate.....
(ii) Copper(i) oxide.......
(iv) Sulphuric acid........
(v) Zinc sulphate.........
(vi) Aluminium chloride......
(vii) Calcium nitride.....
(viii) Hydrogen sulphide......
(ix) Iron(iii) oxide....
(x) Manganese(iv) oxide.....
it's urgent
Answers
Answer:
Na2SO4KNO3Cu2O.H2SO4. ZnSO(AlCl3)Ca3N2H²SFe2O3MnO²PLEASE MARK ME BRAINLIEST.Related Questions
Mike Trout of the Los Angeles Angels has a contract worth $426,500,000. If you earn $100,000 per year, how many years would it take for you to earn what Mike Trout will earn from his contract?
Answers
Answer:
The number of years it would take you to earn what Mike Trout will earn on his contract is 4,265
Explanation:
Mike Trout of the Los Angeles Angels has a contract worth $426,500,000
On the other side, you earn $100,000 per year.
To calculate the number of years it would take for you to earn what Mike Trout will earn from his contract, you must divide the amount Mike earned by the amount you earned during a year:
$426,500,000 ÷ $100,000 per year= 4,265
The number of years it would take you to earn what Mike Trout will earn on his contract is 4,265
Who believed in the caloric theory?
Answers
Lavoisier believed in the caloric theory.
What did scientists believe about caloric?Caloric was a self-repelling fragment that adhered to matter. The heat was a mysterious fluid stored in the matter and liberate during burning and chemical reactions. Heat walk to cold. Hot objects had caloric and cold had little caloric.
The caloric theory is a disused scientific theory that heat consists of a self-resistant fluid called caloric that flows from hotter bodies to colder bodies. Caloric was also the notion of a weightless gas that could pass in and out of pores in solids and liquids.
So we can conclude that Caloric theory The caloric theory is an outworn scientific theory that heat consists of a fluid called caloric.
Learn more about caloric here: https://brainly.com/question/1061571
#SPJ1
undergoing nucleophilic acyl substitution with methanol under reflux conditions to form butanoic acid with a methyl ester substituent on carbon 4. Which structure was most likely the starting CA derivative
Answers
Under acidic or basic circumstances, carboxylic acid (CA) derivatives can undergo nucleophilic acyl substitution. Therefore nucleophilic acyl substitution is most likely the starting CA derivative.
A reaction known as nucleophilic acyl substitution occurs when a nucleophile creates a new bond with an acyl group's carbonyl carbon while simultaneously rupturing the bond between the carbonyl carbon and a leaving group. Due to the fact that a bond is being formed and broken on the same carbon, this reaction is categorised as a substitution.
Anhydrides undergo nucleophilic acyl substitution and are cleaved into two molecules: A carboxylic acid and either an ester or amide, if the nucleophile is an alcohol, or an amine, respectively.
To learn more about substitution
https://brainly.com/question/29560851
#SPJ4
In an electrolytic cell, which ion would migrate
through the solution to the negative electrode?
(1) a chloride ion
(3) a bromide ion
(2) a silver ion
(4) a fluoride ion
Answers
Answer:
Silver ion
Explanation:
In an electrolytic cell, the positive ions known as the cations migrate to the negative electrode, the cathode where they accept electrons to become discharged as neutral atoms. Also, the negative ions known as the anions migrate to the positive electrode, the anode, where they give up their electrons to become discharged as neutral atoms.
From the question above, the only positive ion among the options given is the silver ion, Ag+, therefore, it is the correct option.
If 36. 0 ml of 0. 20 m hcl is added to 30. 0 ml of 0. 40 m naoh, what will be the ph of the resulting solution?
Answers
The pH of the resulting solution is 2.05
Sodium hydroxide and hydrochloric acid neutralize each other in a 1:1 mole ratio as described by the balanced chemical equation
\(NaOH_{(aq)} + HCL_{(aq)}\) → \(NaCl_{(aq)} + H_2O_{(aq)}\)
This means that a complete neutralization, which would result in a neutral solution, i.e. a solution that has pH=7 at room temperature, requires equal numbers of moles of sodium hydroxide and hydrochloric acid.
Notice that your two solutions have equal molarities, but that the volume of the hydrochloric acid solution is 1.2 times larger than the volume of the sodium hydroxide solution. This implies that the number of moles of hydrochloric acid is 1.2 times bigger than the number of moles of sodium hydroxide.
This means that after the reaction is complete, you will be left with excess hydrochloric acid → the pH of the resulting solution will be <7.
Now, the number of moles of hydrochloric acid that will not take part in the reaction is given by
= moles of HCL added - moles of NaOH added
= 36 × \(\frac{0.100 moles HCL}{10^3 ml}\) - 30 × \(\frac{0.100 moles HCL}{10^3 ml}\)
= \(\frac{6* 0.100 moles}{10^3}\)
The total volume of the resulting solution will be = ( 30 + 36 ) mL
= 66 mL
Since hydrochloric acid is a strong acid that ionizes in a 1:1 mole ratio to produce hydronium cations, you can say that the concentration of hydronium cations in the resulting solution will be
\([ H_3O^+] = \frac{\frac{6*0.100}{10^3} }{66*10^3}\)
\([ H_3O^+] = \frac{6*0.100}{66}\)
pH = - log \((\frac{6*0.100}{66} )\)
pH = 2.05
Therefore, the pH of the resulting solution is 2.05.
Learn more about pH here:
https://brainly.com/question/22390063
#SPJ4
Consider the following chemical equation.
2H2 + O2 +2H20
Answers
Answer:
a) mass of H₂O produced = 4.0665 * 18 = 73.197 g
b)mass of H₂O produced = 0.214 * 18 = 3.852 g
c) Oxygen gas, O₂, is the limiting reactant, since the number of moles available for reaction is far smaller and the mass of water produced from it is smaller too.
The reaction is the combustion of hydrogen gas to form water vapor. The question is not complete. A related question is given below:
Consider the following balanced equation:
2H2 + O2 --------> 2H2O
If you start with 8.133 g of H2 and 3.425 g of O2, find the following:
a) With excess O2, what mass (grams) of H2O would be produced by the H2?
b) With excess H2, what mass (grams) of H2O would be produced by the O2?
c) What is the chemical formula for the limiting reactant?
Explanation:
Equation of reaction: 2H₂ + O₂ ---> 2H₂O
From the equation of reaction, 2 moles of hydrogen gas reacts with 1 mole of oxygen gas to produce two moles of water
Molar mass of hydrogen gas, H₂ = 2 g/mol; molar mass of oxygen gas, O₂ = 32 g/mol; molar mass of H₂O = 18 g/mol
a) number of moles of hydrogen in 8.133 g = 8.133 g/ 2g/mol = 4.0665 moles
mole ratio of H₂ to H₂O is 1:1, therefore, 4.0665 moles of H₂O will be produced
mass of H₂O produced = 4.0665 * 18 = 73.197 g
b) number of moles of oxygen in 3.425 g = 3.425 g /32 g/mol = 0.107 moles
mole ratio of O₂ to H₂O is 1:2, therefore, 0.107 * 2 moles of H₂O will be produced = 0.214 moles of H₂O
mass of H₂O produced = 0.214 * 18 = 3.852 g
c) number of moles of hydrogen in 8.133 g = 4.0665 moles
number of moles of oxygen in 3.425 g = 0.107 moles
mole ratio of H₂ to O₂ = 4.0665/0.107 = 38 : 1
Oxygen gas, O₂, is the limiting reactant, since the number of moles available for reaction is far smaller and the mass of water produced from it is smaller too.
Calculate the volumes of the samples used in procedure step 2. Record the calculated volumes in table 2.2 why would we not find the volume of the bolt by this method

Answers
For the bar the calculate volume is:
\(V=l\times w\times h=9.7\text{ cm }\times1.6\text{ cm }\times1.0\text{ cm = 15.5 cm}^3\)For the rod the calculate volume is:
\(V=\pi\times r^2\times h\)\(V=\pi\times(\frac{}{}\frac{1.7}{2}cm)^2\times10.5\text{ cm = 23.8 cm}^3\)kingsley then adds 46.14 ml of naoh to 250.00 ml of the hcooh solution. the neutralization reaction resulted in 0.083 moles of hcooh and 0.037 moles of hcoo- left in solution. determine the ph of the resulting solution.
Answers
If kingsley adds 46.14 ml of NaOH to 250.00 ml of the HCOOH solution. the neutralization reaction resulted in 0.083 moles of HCOOH and 0.037 moles of\(HCOO^-\) left in solution The pH of the resulting solution is 2.21.
The balanced chemical equation for the neutralization reaction between HCOOH (formic acid) and NaOH (sodium hydroxide) is:
HCOOH + NaOH → NaCOOH + \(H_2O\)
From the balanced equation, we can see that 1 mole of HCOOH reacts with 1 mole of NaOH to produce 1 mole of NaCOOH and 1 mole of water.
First, we need to calculate the amount of HCOOH that reacted with NaOH:
0.083 moles HCOOH - 0.037 moles \(HCOO^-\) = 0.046 moles HCOOH
This is the amount of HCOOH that reacted with the 46.14 ml of NaOH added. We need to convert the volume of NaOH to moles using its molarity:
Molarity of NaOH = moles of NaOH / volume of NaOH (in liters)
Assuming the molarity of NaOH is 1.00 M (which is a common concentration for laboratory use), we have:
1.00 M = moles of NaOH / 0.04614 L
moles of NaOH = 0.04614 L × 1.00 M = 0.04614 moles
Since the reaction between HCOOH and NaOH is a 1:1 reaction, the number of moles of HCOOH left in solution is:
0.083 - 0.04614 = 0.03686 moles
To calculate the concentration of HCOOH, we divide the number of moles by the total volume of the solution:
0.03686 moles / 0.25000 L = 0.14744 M
Next, we need to calculate the concentration of HCOO-:
0.037 moles / 0.25000 L = 0.148 M
Since HCOOH is a weak acid, it partially dissociates in water to form \(H^+\) and \(HCOO^-\) ions. The equilibrium constant for this reaction is:
\(Ka = [H^+][HCOO^-] / [HCOOH]\)
We can assume that the concentration of \(H^+\) ions is equal to the concentration of \(HCOO^-\) ions, since HCOOH is a weak acid and does not dissociate much. We can also assume that the concentration of \(HCOO^-\) is equal to the amount of \(HCOO^-\) left in solution (0.037 moles / 0.25000 L = 0.148 M).
Therefore, we can simplify the expression for Ka to:
\(Ka = [HCOO^-]^2 / [HCOOH]\)
Solving for [H+]:
\([H^+] = \sqrt{(Ka * [HCOOH] / [HCOO^-])}\)
The Ka value for formic acid is \(1.8 * 10^{-4\). Substituting the values:
\([H^+] = \sqrt{(1.8 * 10^{-4} * 0.14744 M / 0.148 M)} = 0.00618 M\)
Taking the negative logarithm of [H+] gives us the pH of the solution:
\(pH = -log[H^+] = -log(0.00618) = 2.21\)
For more question on neutralization reaction click on
https://brainly.com/question/23008798
#SPJ11
How many liters of carbon dioxide can be “scrubbed”, or removed, with 3.45 Liters of 0.10 M of lithium hydroxide solution?
Answers
Answer:
The correct answer is - 3.864 L of CO2.
Explanation:
a set of dilutions ranging in concentration from 1x10-2 m to 2x10-2 m are used to prepare a standard curve. transmittances of the dilutions range from 12% to 88%. could this plot be used to determine the concentration of a sample that had a concentration of 1.0x10-3 m?
Answers
The correct option is (B) No, the solution is too dilute and would have a %T over 90% where Beer's Law does not apply.
The solution is too dilute, it will have lower than desired concentration of the desired substance. To make solution more concentrated, more of the desired substance must be added to the solution. This increases the concentration of the desired substance, allowing it to be more effective in the intended application.
The amount of the desired substance must be adjusted according to the desired concentration of the solution. In addition, the amount of other substances in the solution must be considered to ensure the desired concentration is not too high or too low. Too high concentration could produce an undesired reaction, while too low a concentration would decrease the efficiency of the desired reaction.
Full question:
A set of dilutions ranging in concentration from 1x10-2 M to 2x10-2 M are used to prepare a standard curve. Transmittances of the dilutions range from 12% to 88%. Could this plot be used to determine the concentration of a sample that had a concentration of 1.0x10-3 M?
A) No, the solution is too dilute and would have a %T under 10% where Beer's Law does not apply.
B) No, the solution is too dilute and would have a %T over 90% where Beer's Law does not apply.
C) Yes, simply extend the standard curve.
D) No, the solution is too concentrated and would have a %T over 90% where Beer's Law does not apply.
To know more about Dilute solution:
brainly.com/question/16926361
#SPJ4
Which is a positive effect of using chemistry?
Answers
Answer: Chemistry will help us solve many future problems, including sustainable energy and food production, managing our environment, providing safe drinking water and promoting human and environmental health.
Explanation: I Know... hope this helps
Answer:
Chemistry will help us to solve many future problems ,including managing our environment and promoting human.
imagine you are frosting a cake apply pascal's law to using the bag of frosting what would happen when you squeeze the bag
Answers
PLEASE HELP. What is the original (LiOH] if the equivalence point of a titration is reached when 35.5
mL of 0.40 M HBr is added to 25.0 mL of LiOH?
A. 0.57 M B. 0.28 M. C. 0.014 M D. 0.024 M
Answers
Answer:
hagaowtw8twbwjwyw kwuwutw6wt2ywvw witw6wyvwa86w6wcwe ue5wt3yfwywyw iwywiw7w
uwiuwwj9ww
Explanation:
iwjwyaab77w92 and a wherever whatever wueetwb eiw
uwyiwgwkewbekkwuw jwywue kwuwiwywh
kwywiwyw iwtwigw eiywgwiw it wow
bwwneo
Jamie works at a company that makes
cleaning chemicals. She is trying to make
a chemical that smells like flowers. She
took two samples that were gases at room
temperature and mixed them in a sealed
container. The diagram above shows the
repeating groups of atoms that make up the
two starting substances.
After mixing, Jamie found two substances
that smelled like flowers in the sealed
container. (Nothing had escaped.) Which of
the diagrams to the left show the repeating
groups of atoms that make up the ending
substances?
Answers
Answer:
C
Explanation:
Because i got a 100% on it
In the Born-Haber cycle for NaCl(s), which of the following processes corresponds to the enthalpy of formation of NaCl(s)?
a. Na+(aq) + Cl^-(aq) → NaCl(s)
b. 2Na(g) + Cl2(g) → 2NaCl(g)
c. NaCl(g) → NaCl(s)
d. Na(s) + 1/2Cl2(g) → NaCl(s)
e. NaCl(aq) → NaCl(s)
Answers
In the Born-Haber cycle for NaCl(s), Na(s) + 1/2Cl₂(g) → NaCl(s) corresponds to the enthalpy of formation of NaCl(s). The correct option is d.
Born-Haber cycle for NaCl(s)The Born-Haber cycle is the process of estimating the lattice enthalpy of ionic compounds such as NaCl.
A Born-Haber cycle is an energy cycle that calculates the energy involved in each stage of the formation of an ionic compound from its constituent elements. It is named after Max Born and Fritz Haber.
The Born-Haber cycle for NaCl(s) is shown below:
The answer is option D: Na(s) + 1/2Cl₂(g) → NaCl(s)
The formation of NaCl(s) is based on Hess’s Law of Constant Heat Summation. It represents the enthalpy change in a chemical reaction that is independent of the path taken to go from reactants to products.
To obtain NaCl(s), it is necessary to convert the respective elements to the appropriate form. Na(s) is derived from its liquid state, while Cl₂(g) is derived from its gaseous state. NaCl(s) is obtained from the combination of Na(s) and Cl₂(g).
Thus, the enthalpy of formation of NaCl(s) corresponds to the reaction:
Na(s) + 1/2Cl₂(g) → NaCl(s)
Hence, option D is the correct answer.
To know more about Born-Haber cycle, refer to the link below:
https://brainly.com/question/31463853#
#SPJ11
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 4.1 g of butane is mixed with 22.7 g of oxygen. calculate the maximum mass of water that could be produced by the chemical reaction. round your answer to significant digits.
Answers
To determine the maximum mass of water that could be produced in the chemical reaction between gaseous butane and gaseous oxygen, we need to use stoichiometry.
1. Write the balanced chemical equation for the reaction:
C₄H₁₀ (butane) + O₂ (oxygen) → CO₂ (carbon dioxide) + H₂O (water)
2. Determine the molar masses of the reactants and products:
Molar mass of butane (C₄H₁₀) = 4 * (12.01 g/mol) + 10 * (1.01 g/mol) = 58.12 g/mol
Molar mass of oxygen (O₂) = 2 * (16.00 g/mol) = 32.00 g/mol
Molar mass of carbon dioxide (CO₂) = 12.01 g/mol + 2 * (16.00 g/mol) = 44.01 g/mol
Molar mass of water (H₂O) = 2 * (1.01 g/mol) + 16.00 g/mol = 18.02 g/mol
3. Calculate the moles of butane and oxygen:
Moles of butane = mass of butane / molar mass of butane = 4.1 g / 58.12 g/mol = 0.0705 mol
Moles of oxygen = mass of oxygen / molar mass of oxygen = 22.7 g / 32.00 g/mol = 0.7094 mol
4. Use the balanced equation to determine the mole ratio between butane and water:
From the balanced equation, we can see that the mole ratio between butane and water is 1:2.
5. Calculate the moles of water produced:
Moles of water = 2 * moles of butane = 2 * 0.0705 mol = 0.141 mol
6. Determine the mass of water produced:
Mass of water = moles of water * molar mass of water = 0.141 mol * 18.02 g/mol = 2.54 g
Therefore, the maximum mass of water that could be produced by the chemical reaction is 2.54 grams, rounded to significant digits.
Learn more stoichiometry here:
https://brainly.com/question/28780091
#SPJ11
erface/acelus einemClassiD-12683046
The system below was at equilibrium and
then some SO3 gas was removed from the
container. What change will occur for the
system?
2SO2(g) + O₂(g) = 2SO3(g) + 198 kJ
Answers
When some \(SO_3\) gas is removed from the container, the system responds by shifting the equilibrium towards the forward reaction, resulting in an additional production of \(SO_3\) to restore equilibrium.
Le Chartelier's principleWhen some \(SO_3\) gas is removed from the container, the equilibrium of the system will be disturbed. According to Le Chatelier's principle, the system will respond to counteract the change and restore equilibrium.
In this case, by removing \(SO_3\) gas from the container, the concentration of \(SO_3\) will decrease. To restore equilibrium, the reaction will shift in the forward direction to produce more \(SO_3\) gas.
This means that more \(SO_2\) and \(O_2\) will react to form additional \(SO_3\). The forward reaction is exothermic, so it will also help to offset the removal of heat caused by the decrease in \(SO_3\) concentration.
More on Le Chartelier's principle can be found here: https://brainly.com/question/29009512
#SPJ1
An analytical chemist has determined by measurements that there are 2.4 moles of carbon in a sample of acetic acid. how many moles of hydrogen are in the sample?
Answers
Analytical chemist analyses show that a sample of acetic acid contains 2.4 moles of carbon. The number of moles of hydrogen in the sample is 5.75 moles.
What are moles?The mole is a SI unit of measurement that is used to calculate the quantity of any substance. Mole can be calculated by dividing the mass by the molar mass.
Acetic acid is a colorless liquid, with a pungent smell.
The formula of acetic acid is CH₃COOH
For every 12 moles of hydrogen, there's 1 mole of oxygen.
2.4 / 12 = 0.2.
Thus, therefore, there are 5.75 moles of oxygen in the sample.
To learn more about moles, refer to the link:
https://brainly.com/question/26416088
#SPJ4
Although ATP is the main energy currency in cells, other molecules, such as NAD, play a central role in some metabolic pathways by transferring electrons. The oxidized form of NAD is NAD+, and the reduced form is NADH. Identify the components of NAD+ and ATP. NH, O=P 0 NH, N OH OH O 0 NH 0 0 0 O=P-0 OH OH OH OH ATP NAD Answer Bank deoxyribose phosphate adenine nicotinamide ribose Select the components that are common to both ATP and NAD. ribose adenine deoxyribose phosphate nicotinamide
Answers
The components that are common to both ATP and NAD are: adenine and ribose. Adenine and ribose are both found in ATP and NAD molecules.
What are ATP and NAD?ATP stands for Adenosine Triphosphate, which is the primary energy carrier in cells. ATP is an energy-rich molecule that stores energy that can be used by the cell. It is composed of three phosphate groups, an adenine base, and a ribose sugar.
NAD stands for Nicotinamide Adenine Dinucleotide, which is an electron carrier molecule that is involved in many cellular metabolic reactions. It is composed of two nucleotides (adenine and ribose) linked by two phosphate groups.
The oxidized form of NAD is NAD+ while the reduced form is NADH.
The components of ATP and NAD are: Adenine and ribose are the two components common to both ATP and NAD.
Other components are specific to each molecule, as follows: ATP components: Three phosphate groups An adenine base A ribose sugar NAD+ components: Nicotinamide (a type of vitamin B3)Adenine A ribose sugar Two phosphate groups.
To learn more about "ATP" here:
https://brainly.com/question/30387542#
#SPJ11
The element x has three naturally occurring isotopes. the masses (amu) and bundances of the isotopes are given in the table below. the average atomic mass of the element is ________ amu.
Isotope Abundance(%) Mass(amu)
15X 28.6 15.33
17X 13.3 17.26
16X 58.1 18.11
A. 17.20
B. 16.90
C.17.65
D. 17.11
Answers
The average atomic mass of the element is 17.20 amu. This is calculated using the relative abundances and atomic masses of its isotopes.
In order to calculate the average atomic mass of the element, we need to multiply the relative atomic mass of each isotope by its abundance, and then add them up.
So, for isotope ¹⁵X:
15.33 amu * 0.286 = 4.38 amu
For ¹⁷X:
17.26 amu * 0.133 = 2.30 amu
And for ¹⁸X:
18.11 amu * 0.581 = 10.52 amu
Now, finally, we add these up to get the average atomic mass of element X:
4.38 amu + 2.30 amu + 10.52 amu = 17.20 amu
You can learn more about the average atomic mass here:
brainly.com/question/13753702
#SPJ4
100.0 mL of 3.8M NaCN, the minimum lethal concentration of sodium cyanide in blood serum
Answers
The given question is incomplete. The complete question is:
Calculate the number of moles and the mass of the solute in each of the following solution: 100.0 mL of 3.8 × 10−5 M NaCN, the minimum lethal concentration of sodium cyanide in blood serum
Answer: The number of moles and the mass of the solute are \(0.38\times 10^{-5}\) and \(18.62\times 10^{-5}g\) respectively
Explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
\(Molarity=\frac{n\times 1000}{V_s}\)
where,
n = moles of solute
\(V_s\) = volume of solution in ml
\(3.8\times 10^{-5}M=\frac{n\times 1000}{100.0}\)
\(n=0.38\times 10^{-5}\)
n = moles of \(NaCN\) = \(\frac{\text {given mass}}{\text {Molar mass}}\)
\(0.38\times 10^{-5}=\frac{x}{49g/mol}\)
\(x=18.62\times 10^{-5}g\)
Thus the number of moles and the mass of the solute are \(0.38\times 10^{-5}\) and \(18.62\times 10^{-5}g\) respectively
Question 3 please help :)

Answers
Explanation:
Removing B from the system
- Decreases the rate of the reaction. Backward reaction (formation of reactants) is favoured.
Crushing A into a powder
- Increases the rate of reaction. This is because of the increased surface area of A.
Warming the system
- Increases the rate of the reaction. Temperature is proportional to rate of reaction.
Adding more A to the system
- Increases the rate of reaction. Forward reaction (formation of products) is favoured.
Putting the system into an ice bath
- Decreases the rate of reaction. Temperature is proportional to rate of reaction.
Decreasing the pressure of the system
- Decreases the rate of the reaction.
how many meals would you get if you were to go on a flight to mars from earth
Answers
Each crew member receives three wholesome meals daily along with snacks. The food for each astronaut is kept onboard the Shuttle and is given a unique color dot to identify it.
This is further explained below.
What is Mars?Generally, Being bigger than only Mercury, Mars is the second-smallest planet in the Solar System and is located four planets from the Sun. The Roman god of battle is the inspiration for the name of Mars in English.
It provides each member of the crew with three meals that are well-balanced, in addition to snacks. On board the Space Shuttle is where all of the astronauts' food is kept, and each individual box has a colorful dot that identifies it.
In conclusion, For example, traveling to Mars and returning may take more than three years and need the supply of thousands of kg of food. If the crew of four were to consume merely three meals per day while on their three-year voyage to Mars, they would need to bring more than 24,000 pounds (10,886 kilograms) of food with them.
Read more about mars
https://brainly.com/question/14623285
#SPJ1
Temperature is one of the conditions that affects both physical and chemical change. Support the statement with an example.
Answers
Temperature affects both physical and chemical change because it modifies the state of matter and also it alters the rate of chemical reactions.
How does temperature affect physicochemical properties?Temperature affects physical properties by increasing the motion of constituent atoms and thus altering the state of matter.
Moreover, in a chemical reaction, an increase in temperature also affects the conversion rate of a reactant to one or more products.
In conclusion, temperature affects both physical and chemical change because it modifies the state of matter and also it alters the rate of chemical reactions.
Learn more about temperature effects here:
https://brainly.com/question/17928400
#SPJ1
Calculate the pressure in atm needed to compress 1 kilogram of water from volume 1.00litre to volume 0.99 litre.Hint: You will need to use the bulk modulus for water: B=2.0x10^9 Pa
Answers
The pressure needed to compress 1 kilogram of water from volume 1.00 litre to volume 0.99 litre is 197 atm.
To calculate the pressure needed to compress 1 kilogram of water from volume 1.00 litre to volume 0.99 litre, we can use the formula for bulk modulus:
B = -V(dp/dV)
where B is the bulk modulus, V is the initial volume, dp is the change in pressure, and dV is the change in volume.
We can rearrange this formula to solve for dp:
dp = -(B/V) * dV
Substituting the given values, we get:
dp = -(2.0x10^9 Pa / 1.00 L) * (0.01 L)
dp = -2.0x10^7 Pa
Since we want to find the pressure needed to compress the water, we need to use the negative of this value:
dp = 2.0x10^7 Pa
Finally, we can convert this pressure to atm by dividing by the standard atmospheric pressure of 1 atm:
pressure = dp / (1 atm / 101325 Pa)
pressure = 197 atm
To know more about pressure and compression : https://brainly.com/question/28012687
#SPJ11
Witch statement about greenhouse gases is false
Answers
Answer:
???
Explanation:
Examine the diagram shown. Which are the correct labels for continental plates 1 and 2?
2
equator
1
1 = Antarctic, 2 = African
1 = Indo-Australian, 2 = Eurasian
1 = North American, 2 = Pacific
1 = South American, 2 = Antarctic
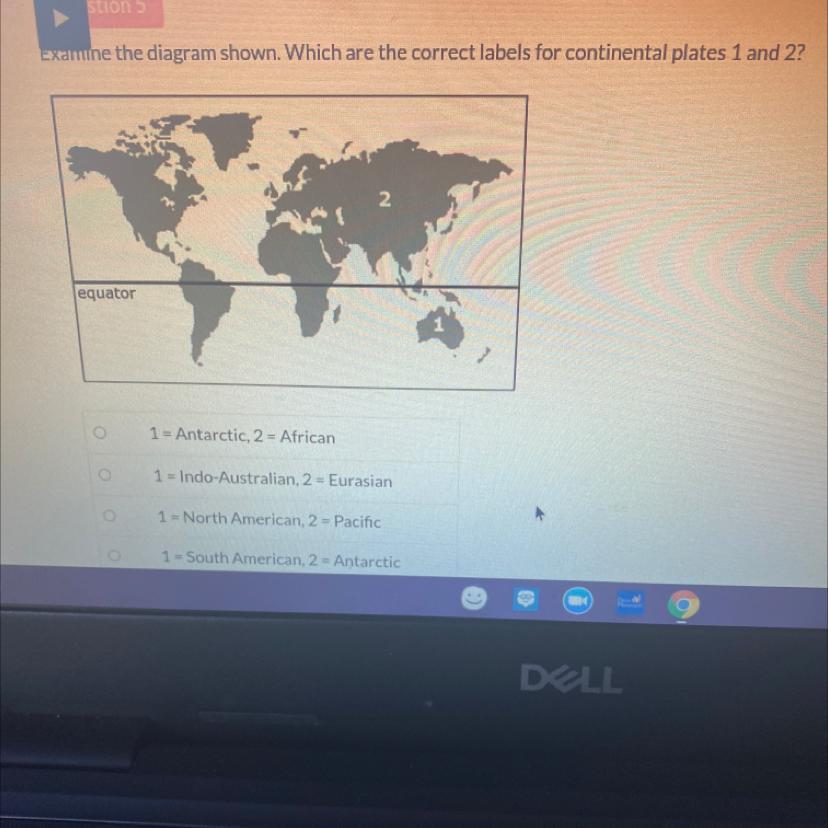
Answers
Answer:
Australian and eurasian
Explanation:
How would you prepare a 35 ml solution of 95% (volume/volume solution of ethanol?
Answers
Explanation:
Solutions. 1. If 47 g of KCl dissolved in enough water to give 375 mL of soloution, what is the molarity ... vo volume of solute . ... v/v ethanol, how much 95% v/v ethanol ... prepare 200. mL ...
How many atoms are in 9.3moles of lithium?
Answers
Answer:
3.45 moles Li contains 2.08 × 10 (to the power of)24 atoms .
Explanation:
The relationship between atoms and moles is:
1 mole atoms =
6.022 × 10 (to the power of)23
atoms
In order to determine how many atoms occupy a given number of moles, multiply the given moles by
6.022 × 10 (to the power of)23
atoms/mole
.
In the case of 3.45 moles lithium (Li):
3.45 mol Li × 6.022 × 10 (to the power of)23 atoms Li/ 1 mol Li =
2.08 × 10 (to the power of)24
atoms Li rounded to three
Answer: 3.45 moles Li contains 2.08 × 10 (to the power of)24 atoms
Explanation:
A/An _______ indicates the ratio of atoms in a molecule.
Answers
Answer:
A molecular formula indicates the ratio of atoms in a molecule.
Explanation:
A molecular formula or chemical formula is a representation or indication of the number of atoms in a particular compound or molecule have in a specific specific proportion.
These are represented by the symbols of elements, numbers of atoms present and sometime other symbols. For example in water molecule there is one atom of oxygen and two hydrogen atoms.
Thus, the correct answer is : molecular formula