5. Stellar equilibrium relies on fusion and gravity to maintain stars in their current form. (10 points) A. What will the sun turn into when it runs out of fuel for fusion? (5 points) B. Do you think this will mean the end of life on Earth? (5 points) 6. The SETI project constantly is searching for life on other planets. A. Given what you know about the size of the universe, do you support the hypothesis that there is life on other planets? Why or why not? (5 points) B. Why is it difficult to find evidence of life on other planets? (5 points)
Answers
Stellar equilibrium relies on fusion and gravity to maintain stars in their current form.
What is the Stellar equilibrium?5A. When the sun runs out of fuel for fusion, it will evolve into a red giant star, which will eventually shed its outer layers and become a white dwarf.
B. No, the end of the sun's life as a white dwarf will not mean the end of life on Earth. By the time the sun runs out of fuel for fusion, life on Earth will have ceased to exist long before that due to other factors like the gradual increase in solar luminosity over time.
6. The SETI project constantly is searching for life on other planets.
A. Given the vastness of the universe, it is highly likely that there is life on other planets. The universe contains billions of galaxies, each containing billions of stars and planets. It is probable that some of these planets have the necessary conditions to support life.
Therefore, for question B. It is difficult to find evidence of life on other planets because of the vast distances involved. Even the closest star to our solar system, Proxima Centauri, is over 4 light years away. This means that any signals or evidence of life would take years to reach us, and any attempts at communication would also take years to receive a response.
Read more about Stellar equilibrium here:
https://brainly.com/question/6373350
#SPJ1
Related Questions
help heating curve iron
at what temperature does the substance begins to boil
at what temperature does a substance begin to melt
at what temperature is a substance for a liquid and a gas
at what temperature is the substance both a solid and a liquid

Answers
At 2861 degree Celsius the iron begins to boil. At 1,538 °C the substance begins to melt.
The melting point is the point at which the liquid and solid forms of a solid can exist in equilibrium. It can also be defined as the point at which a solid changes into a liquid under normal atmospheric pressure.
The equilibrium point at which water vapor, liquid water, and solid ice can exist in equilibrium is the only point at which the pressure and temperature of water vapor are the same. The equilibrium point of water vapor is the point at which the partial vapor pressure is the same as that of liquid water at the exact temperature of 273.1600 K.
To learn more about iron, refer to the link:
https://brainly.com/question/31984794
#SPJ1
A sample of helium occupies a volume of 160cm3 at 100 KPa and 25°c. what volume will it occupy if the pressure is adjusted to 80 KPa and the temperature remains unchanged?
Answers
Answer:
Explanation:Explore this page
About the gas laws calculator
This is an ideal gas law calculator which incorporates the Boyle's law , Charles's law, Avogadro's law and Gay Lussac's law into one easy to use tool you can use as a:
Boyle's Law-
\(\:\:\:\:\:\:\:\:\:\:\:\star\:\sf \underline{ P_1 \: V_1=P_2 \: V_2}\\\)
(Pressure is inversely proportional to the volume)
Where-
\(\sf V_1\) = Initial volume\(\sf V_2\) = Final volume\(\sf P_1\) = Initial pressure\(\sf P_2\) = Final pressureAs per question, we are given that -
\(\sf V_1\) = 160 cm³\(\sf P_1\) = 100KPa\(\sf P_2\) = 80KPaNow that we have all the required values and we are asked to find out that volume which will be occupied if the pressure is adjusted to 80 KPa and the temperature remains unchanged. For that we can put the values and solve for the final volume of helium-
\(\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\star\:\sf \underline{ P_1 \: V_1=P_2 \: V_2}\)
\(\:\:\:\: \:\:\:\:\:\:\longrightarrow \sf 100 \times 160 = 80 \times V_2\\\)
\( \:\:\:\:\:\:\:\:\:\:\longrightarrow \sf V_2 = \dfrac{100 \times 160}{80}\\\)
\( \:\:\:\:\:\:\:\:\:\:\longrightarrow \sf V_2 =100\times \cancel{\dfrac{ 160}{80}}\\\)
\(\:\:\:\: \:\:\:\:\:\:\longrightarrow \sf V_2 = 100 \times 2\\\)
\( \:\:\:\:\:\:\:\:\:\:\longrightarrow \sf \underline{V_2 = 200 \:cm^3 }\\\)
Therefore, 200 cm³ will be occupied if the pressure is adjusted to 80 KPa and the temperature remains unchanged.Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
Write the atomic symbols for isotopes with the following characteristics34 protons and 45 neutronsExpress your answer as an isotope.
Answers
Answer and explanation:
Isotopes have the same Atomic number (that's why the have the same number of protons), but different Mass number (that's why they have different number of neutrons).
So, to find the isotope, we have to look for in the Periodic Table of Elements, the element with the same number of protons (34), and we can find it by its Atomic number.
The atom with Atomic number 34 is Selenium with a Mass number of 79, and its symbol is:
\(Se^{79}_{34}^\)An isotope of Selenium could be:
\(\begin{gathered} Se^{78}_{34} \\ . \\ Se_{34}^{80} \end{gathered}\)\(\begin{gathered} \\ \end{gathered}\)78Se is an isotope of Selenium with 44 neutrons and 80Se is an isotope of Selenium with 46 neutrons.
A student wants to help preserve coral reefs which field of study would be most useful ?
Answers
For a student wishing to contribute to the preservation of coral reefs, oceanography would be the most beneficial topic of study because it is ocean-focused.
What are coral reefs?An underwater environment known as a coral reef is characterized by corals that construct reefs. Coral polyp colonies are bound together by calcium carbonate to build reefs. Stony corals, whose polyps gather together, make up the majority of coral reefs.
Coral reefs offer chances for recreation, serve as a barrier against erosion and storm damage, and support local economies. They are also a source of fresh medications and food. More than 500 million people rely on reefs for safety, income, and food.
Learn more about coral reefs here:
https://brainly.com/question/10970167
#SPJ1
please help!!
which oneeeee

Answers
How many liters of .3M HCl are needed to neutralize 2.5L of 3M NaOH?
Answers
Answer: 2.5 lit
Explanation:
Fill in the coefficients that will balance the following reaction: (Note: Use 1 as coefficient where appropriate.) NaCl + CaS → Na2S + CaCl2
Answers
Answer:
the answer is
2,1,1,1
Explanation:
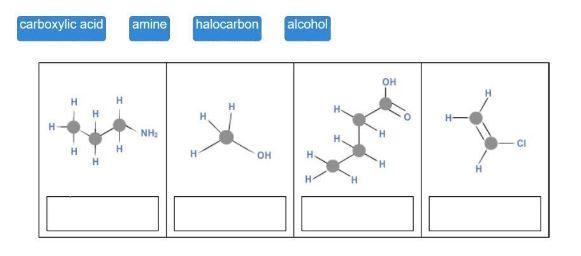
Match each hydrocarbon class to its structure.
4
carboxylic acid
H
H
HT
H
H
H
amine
NH₂
halocarbon
H
OH
alcohol
H.
H
H.
'H
OH
'H
H-
-CI
Answers
The tile's suggested answers include amine, alcohol, carboxyl group, and halocarbon.
Gasoline is it a hydrocarbon?Hydrocarbons are organic substances comprised of hydrogen and carbon, and include petroleum, methane gas, and coal. Alkanes are both a highly combustible chemical and the main source of fuel in the planet. Its uses include diesel, jet fuel, propane, petrol, and petroleum, to name a few.
What makes it a hydrocarbon?The most fundamental category of organic compounds is referred to as a hydrocarbon. As their name implies, they are exclusively made up of the elements hydrogen and carbon. Atoms surround one or more core carbon atoms in hydrocarbon molecules, which are branching or chain-like in shape.
To know more about Hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ1
The complete question is-
Drag each tile to the correct image. Match each hydrocarbon class to its structure. carboxylic acid amine halocarbon alcohol.
What is the empirical formula for CaCo3?*
What is the empirical formula for CaCo3?
A. CaCO3
D. Ca(CO3)2
B. Caco
E. Ca,C,06
C. Ca2CO3
F. none of these
Answers
This problem is requiring the empirical formula for CaCO₃, which is its molecular formula, and turns out to be equal, this is A. CaCO3 according to the following:
Empirical formulas:In chemistry, molecular formulas show both the actual type and number of atoms in a chemical compound, based on the elements across the periodic table and the subscripts standing for the number of atoms in the compound.
However, the empirical formula is a reduced expression of the molecular one, which shows the minimum number of atoms in a compound after simplifying to the smallest whole numbers.
In such a way, since the given compound is CaCO₃ and both Ca and C have a one as their subscript, it is not possible to simplify any further and therefore the empirical formula equals the molecular one this time, making the answer to be A. CaCO3.
Learn more about empirical formulas: https://brainly.com/question/1247523
How would you do this??

Answers
Explanation:
A lot of the names you're just going to have to look up/know. CH4 is methane, which you would just have to know.
As for the molar mass, for each compound you have to add up the masses of each atom. Using CH4 as an example, you would have to add the mass of the carbon atom with the 4 hydrogen atoms. Using a periodic table you can see that the mass of carbon is about 12.0107 g and the mass of each hydrogen atom is about 1.008 g.
12.0107 + (4 x 1.008) = 16.0427 g
Just be careful with compounds such as Ca3(PO4)2, because there are 2 phosphorous atoms and 8 oxygen atoms.
For percent composition you have to find how much each element within the compound makes up the compound's mass. Since you will have already calculated the molar mass, you can add up the mass of a particular element within a compound and divide it by the compound's molar mass. Then multiply by 100 to get the percent.
For CH4,
Carbon:
(12.0107/16.0427) x 100 = 74.87 %
Hydrogen:
(4 x 1.008/16.0427) x 100 = 25.13 %
Question 6 (1 point)
If silver sells for $0.87 per gram, what is the value of the silver in the truck?
O a 103.9 million dollars
O b
103.9 dollars
Oc 137.2 million dollars
Answers
Answer: 43 points my bad Cuh I have to get the points
Explanation: :)
Why is gas compressible??
Answers
Answer:
Because large empty spaces are present between gas molecules.
Explanation:
Element R has three isotopes. The isotopes are present in 0.0398, 0.1614, and 0.7988 relative
abundance. If their masses are 191, 180, and 143 respectively, calculate the atomic mass of element
R. (No decimals)
Answers
The atomic mass of element R is 151 (no decimals).
To calculate the atomic mass of element R, we need to consider the relative abundance of each isotope and its corresponding mass. The atomic mass is the weighted average of the masses of all the isotopes, taking into account their relative abundance.
Given:
Isotope 1: Relative abundance = 0.0398, Mass = 191
Isotope 2: Relative abundance = 0.1614, Mass = 180
Isotope 3: Relative abundance = 0.7988, Mass = 143
To calculate the atomic mass, we multiply the relative abundance of each isotope by its mass, and then sum up the results.
Atomic mass = (Relative abundance of Isotope 1 * Mass of Isotope 1) + (Relative abundance of Isotope 2 * Mass of Isotope 2) + (Relative abundance of Isotope 3 * Mass of Isotope 3)
Atomic mass = (0.0398 * 191) + (0.1614 * 180) + (0.7988 * 143)
Calculating the values:
Atomic mass = 7.6098 + 29.0256 + 114.6872
Atomic mass = 151.3226
Rounding to the nearest whole number, the atomic mass of element R is 151.
For more such question on mass. visit :
https://brainly.com/question/24191825
#SPJ8
Calculate the number of grams of platinum in 0.00808 moles Pt.

Answers
Answer:
1.58 g Pt
Explanation:
one mole of platinum weighs 195.09 grams (195.09 g/mol).
to convert moles of platinum to grams, we simply multiply the given moles of platinum by 195.09 to convert it to grams.
0.00808 x 195.09 = 1.576 g
Which of the following is NOT a property of bases? Which of the following is NOT a property of bases? Bases dissolve many metals. Bases turn litmus paper blue. Bases have a slippery feel. Bases have a bitter taste. All of the above are properties of bases.
Answers
Bases dissolve many metals.
Write the chemical formula for this molecule

Answers
The chemical formula for the molecule you provided is C2H5Cl.
In the molecule, the central atom is carbon (C), which is bonded to two hydrogen atoms (H) and one chlorine atom (Cl). The carbon atom forms single bonds with each of the hydrogen and chlorine atoms, resulting in a linear structure.
To write the chemical formula, we start by indicating the number of atoms of each element present in the molecule. In this case, there are two carbon atoms (C2), five hydrogen atoms (H5), and one chlorine atom (Cl1).
Next, we write the symbols for the elements in the order of their appearance. The formula is typically written with the carbon atom first, followed by hydrogen, and then any other elements in alphabetical order. Therefore, the chemical formula for the molecule is C2H5Cl.
The subscripts in the formula indicate the number of atoms of each element in the molecule. In this case, there are two carbon atoms, five hydrogen atoms, and one chlorine atom.
It's important to note that the formula represents the simplest ratio of atoms in the molecule. It does not provide information about the spatial arrangement or bonding pattern of the atoms. Additional structural information, such as the arrangement of atoms in space, would require a more detailed representation, such as a Lewis structure or a three-dimensional model.
for more questions on chemical formula
https://brainly.com/question/21393201
#SPJ8
What type of molecule is acetylacetone?
O A. Ester
O B. Aldehyde
O C. Ketone
O D. Alcohol
Answers
Answer:
c.ketone is the answer.
For most substances the density of a solid form is slightly greater than the density of liquid form water however is different it is slightly less dense in the solid form than it is in liquid form compare water in the solid form with neon argon in oxygen and proposal reason why solid water is less dense liquid water
Answers
Answer:
Answer: When water freezes, water molecules form a crystalline structure maintained by hydrogen bonding. Solid water, or ice, is less dense than liquid water. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart, which lowers the density.
Explanation:
Why do we monitor chinstrap penguins instead of krill?
Answers
Answer:Yes
Explanation:
Because Chinstrap penguins eat krills
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.
When the temperature of a gas increase at constant volume, the pressure of the gas decreases.True or false?
Answers
The given statement is false.
When the temperature of a gas increases at a constant volume, the particles will have more frequent collisions against the wall of their container which will result in the increase of the pressure.
CAN SOMEONE HELP WITH THIS QUESTION?

Answers
The usual enthalpy change for the 200g/7.68 mol of ethyne reaction is C2H6g C2H2g Plus 2H2g - 2,388.5kJ.
What does the letter H mean for chemistry?The quantity of heat released or absorbed during a reaction occurring at constant pressure is known as the enthalpy change.
In thermodynamics, what does H mean?H stands for "enthalpy change," Hf for "system final enthalpy" (i.e., the enthalpy of the byproducts of the system in equilibrium in a chemical reaction), and Hi for "system initial enthalpy" (i.e., the entropy for the reactants in a chemical reaction).
To know more about enthalpy visit:
https://brainly.com/question/16720480
#SPJ1
Which of the following is a TRUE statement?
A) Bond dissociation energies are exothermic.
B) Bond dissociation energies are endothermic.
C) All C-H bonds require the same energy
amount of energy to break.
D) All C-H bonds release the same amount of
energy when formed.
Answers
Bond dissociation energies are endothermic.
What is bond dissociation energy?
The bond dissociation enthalpy can be used to measure the strength of the chemical link between any two species. The bond dissociation energy of a chemical bond is sometimes defined as the enthalpy change of the homolytic fission of the bond at absolute zero, even though it is typically measured as the enthalpy change at standard conditions (298K) (0K).
Hence, bond dissociation energies are endothermic in nature.
To learn more about Bond Energy, here
https://brainly.com/question/26141360
#SPJ1
Drag force is directly proportional to ______. velocity. thrust shorter and faster blades longer and slower blades
Answers
Answer:
Drag force is directly proportional to squared velocity
Explanation:
googl e
Write the empirical formula for c2h6o2
Answers
Answer:c1h3o1
Explanation:
Ahahah I’m getting points nah jp here
C1h3o1
Fill in the blanks
1. Soil is an important - _______resource
2. For soil formation to take place, bare rocks initially need to breakup as a result of -________- process
3 The uppermost layer of earth's crust in which plant grow is called________
Answers
Answer:
1.natural 2.weathering 3.soil
What is plotted on the Y axis
Speed
Distance or time

Answers
Cause if you look on the side it says distance
how many moles of water are there in :100g of H20 (b)1.00×10 rest to power 24 molecules of H20
Answers
Answer:
amole=5.55
b.mole=1.66
Explanation:
a.m=100g,M(H2O)=2+16=18
mole=100÷18=5.55
Polyethylene is 86.0% C and 14.0%
H. Determine the empirical formula of the compound.
Percent to Mass: How many grams of C/and Hare present in 100.0 g?
Answers
The empirical formula of polyethylene can be determined by converting the given percentages of carbon (C) and hydrogen (H) into grams. To find the grams of each element, we assume a 100.0 g sample of polyethylene.
For carbon:
Mass of carbon = 86.0% × 100.0 g = 86.0 g
For hydrogen:
Mass of hydrogen = 14.0% × 100.0 g = 14.0 g
Therefore, in a 100.0 g sample of polyethylene, there are 86.0 grams of carbon and 14.0 grams of hydrogen.
The empirical formula of a compound represents the simplest whole-number ratio of atoms present in the compound. To determine the empirical formula, we need to find the ratio of carbon to hydrogen in terms of moles.
First, we convert the masses of carbon and hydrogen into moles using their respective molar masses. The molar mass of carbon is approximately 12.01 g/mol, and the molar mass of hydrogen is approximately 1.008 g/mol.
Number of moles of carbon = 86.0 g / 12.01 g/mol ≈ 7.162 mol
Number of moles of hydrogen = 14.0 g / 1.008 g/mol ≈ 13.89 mol
Next, we divide the number of moles of each element by the smallest number of moles to get a simplified ratio.
Carbon: Hydrogen ≈ 7.162 mol : 13.89 mol ≈ 1 : 1.939
Since we want to express the ratio in whole numbers, we multiply both sides by 2 to get a whole number ratio.
Carbon: Hydrogen ≈ 2 : 3.878
Rounding to the nearest whole number, we find that the empirical formula of polyethylene is CH₂.
for such more questions on hydrogen
https://brainly.com/question/24433860
#SPJ8