3. Which phase change requires the removal of energy? *
(1 Point)
condensation
evaporation
melting
sublimation
answer quick pls
Answers
Answer:
I'm pretty sure its condensation The energy absorbed causes the molecules to change patterns. hope i helped
Related Questions
if any 1 studying 11th can u plz send me the chemistry notes for chapter 2 STRUCTURE OF AN ATOM lesson
plz send me honestly cause i have exam day after tommorow .so plz send unwanted answer
I BEG U
Answers
Answer:
What
You cant just send notes you also have to say where you are and which book you are using because others are different
how do i tell someone how i feel?
Answers
A 22.0 g sample of quartz, which has a specific heat capacity of 0.730 J•g .°C , is dropped into an insulated container containing 250.0 g of water at 25.0 °C and a constant pressure of 1 atm. The initial temperature of the quartz is 97.1 °C. Assuming no heat is absorbed from or by the container, or the surroundings, calculate the equilibrium temperature of the water. Be sure your answer has the correct number of significant digits.
Answers
Using the principle of conservation of energy, the equilibrium temperature of the water is approximately 23.9 °C.
The equilibrium temperature of the water can be calculated using the principle of conservation of energy. The heat lost by the quartz equals the heat gained by the water.
First, we calculate the heat lost by the quartz:
q_quartz = m_quartz * c_quartz * (T_equilibrium - T_initial)
where
q_quartz is the heat lost by the quartz,
m_quartz is the mass of the quartz (22.0 g),
c_quartz is the specific heat capacity of quartz (0.730 J•g°C), and
T_initial is the initial temperature of the quartz (97.1 °C).
Next, we calculate the heat gained by the water:
q_water = m_water * c_water * (T_equilibrium - T_water_initial)
where
q_water is the heat gained by the water,
m_water is the mass of water (250.0 g),
c_water is the specific heat capacity of water (4.184 J•g°C), and
T_water_initial is the initial temperature of the water (25.0 °C).
Since no heat is absorbed from or by the container or the surroundings, the heat lost by the quartz is equal to the heat gained by the water:
m_quartz * c_quartz * (T_equilibrium - T_initial) = m_water * c_water * (T_equilibrium - T_water_initial)
Now, we plug in the values and solve for T_equilibrium:
22.0 g * 0.730 J•g°C * (T_equilibrium - 97.1 °C) = 250.0 g * 4.184 J•g°C * (T_equilibrium - 25.0 °C)
Multiplying the terms:
16.06 J/°C * (T_equilibrium - 97.1 °C) = 1046 J/°C * (T_equilibrium - 25.0 °C)
Expanding further:
16.06 J/°C * T_equilibrium - 16.06 J/°C * 97.1 °C = 1046 J/°C * T_equilibrium - 1046 J/°C * 25.0 °C
Simplifying:
16.06 J/°C * T_equilibrium - 1563.626 J = 1046 J/°C * T_equilibrium - 26150 J
Rearranging the equation to isolate T_equilibrium:
16.06 J/°C * T_equilibrium - 1046 J/°C * T_equilibrium = 1563.626 J - 26150 J
-1029.94 J/°C * T_equilibrium = -24586.374 J
Dividing both sides by -1029.94 J/°C:
T_equilibrium = (-24586.374 J) / (-1029.94 J/°C)
T_equilibrium ≈ 23.883 °C
Therefore, the equilibrium temperature of the water is approximately 23.9 °C.
To know more about equilibrium temperature, refer to the link :
https://brainly.com/question/31961430#
#SPJ11
What are some potential traits that are needed to become a super athlete?
Answers
Answer: Confidence, strength, potential
Explanation:
\( \huge \boxed{ \fcolorbox{black}{pink}{Answer}}\)
20 Distinguishing Personality Traits of High-Performing Athletes
1. Self Confidence. “Self-Confidence” isn't just a phrase for cheesy motivational posters. ...
2. Strong Sense of Motivation. It takes more than a shiny medal or hefty check to motivate the world's best athletes. ...
3. Inner Desire to Succeed. ...
4. Natural Goal Setter. ...
5. Self-Discipline. ...
6. Optimism. ...
7. Sense of Belonging. ...
8. Natural Leader.
How long would it take to convert 100. 0 grams of solid sodium at 20. 0°C to sodium vapor at 1000. 0°C if the heating rate at a pressure of one atm is 8. 0 kJ?min–1? The melting point of sodium is 97. 8°C; its boiling point is 883°C; its molar enthalpy of fusion is 2. 60 kJ?mol–1; its molar enthalpy of vaporization is 97. 4 kJ?mol–1; and the heat capacities of solid, liquid, and gaseous sodium are 28. 2 J?mol–1?K–1, 30. 8 J?mol–1?K–1, and 20. 8 J?mol–1?K–1, respectively
Answers
To convert 100.0 grams of solid sodium at 20.0°C to sodium vapor at 1000.0°C, the heating rate at a pressure of one atmosphere must be 8.0 kJ/min–1.
First, calculate the amount of moles of sodium, which is 3.99. The heat required for melting and vaporization can be calculated as follows:
Heat for melting = 3.99 moles × 2.60 kJ/mol = 10.37 kJHeat for vaporization = 3.99 moles × 97.4 kJ/mol = 389.06 kJThen, calculate the total heat required to raise the temperature from 20.0°C to 97.8°C and from 97.8°C to 1000.0°C.
Heat for temperature change from 20.0° C to 97.8° C = 3.99 moles × (30.8 J/mol/K × 77.8 K) = 11960.99 JHeat for temperature change from 97.8° C to 1000.0° C = 3.99 moles × (20.8 J/mol/K × 902.2 K) = 81553.66 JFinally, calculate the total heat required to convert 100.0 grams of solid sodium at 20.0°C to sodium vapor at 1000.0°C:
Total Heat = 10.37 kJ + 389.06 kJ + 11960.99 J + 81553.66 J = 91914.08 J
Therefore, it would take 91914.08 J of heat energy to convert 100.0 grams of solid sodium at 20.0° C to sodium vapor at 1000.0°C if the heating rate at a pressure of one atmosphere is 8.0 kJ/min–1.
For more information about the heating rate refers to the link: https://brainly.com/question/13411214
#SPJ11
How is oxide different from a neutral oxygen atom
Answers
Answer: The main difference between oxide and oxygen is that oxide is a chemical compound with at least one oxygen atom while oxygen is an element whose atomic number is 8.
Explanation: let me know if it was right or wrong
which of the following is lewis acid a. Bacl. b.kcl c.Becl2 d.Licl
Answers
Answer:
becl2
Explanation:
because
a it has incomplete outer
b it accept electron pair
Which type of electrons are best at shielding a 3p electron?1) 2p2) 3p3) 4p4) 3s5) 3d
Answers
The type of electrons that are best at shielding a 3p electron is 4) 3s electrons.
Shielding refers to the ability of electrons in inner energy levels to repel or shield outer electrons from the full effect of the positive charge of the nucleus. Electrons in lower energy levels (closer to the nucleus) have a stronger shielding effect on outer electrons.
In this case, the 3p electron is in the outermost energy level. The electrons in the 3s sublevel are in the same energy level as the 3p electron but are closer to the nucleus. Therefore, the 3s electrons have a better shielding effect on the 3p electron compared to the other options listed.
The 2p electrons (option 1) are in a lower energy level, so they have less shielding effect on the 3p electron. The 3p electrons themselves (option 2) do not contribute to the shielding effect. The 4p electrons (option 3) are in a higher energy level and are further away from the nucleus, so their shielding effect is weaker. The 3d electrons (option 5) are in a higher energy level but have less shielding effect compared to the 3s electrons.
Therefore, the 3s electrons (option 4) are best at shielding a 3p electron.
To know more about electrons
brainly.com/question/12001116
#SPJ11
Iron(III) oxide is formed when iron combines with oxygen in the air.
How many grams of Fe203 are formed when 6.7 g of Fe reacts completely with oxygen?
Fe + 02 --> Fe203 (unbalanced equation)
Answers
Answer:
Mass = 9.58 g
Explanation:
Given data:
Mass of Fe₂O₃ formed = ?
Mass of Fe = 6.7 g
Solution:
Chemical equation:
4Fe + 3O₂ → 2Fe₂O₃
Number of moles of Fe:
Number of moles = mass/molar mass
Number of moles = 6.7 g/ 55.8 g/mol
Number of moles = 0.12 mol
now we will compare the moles of Fe and Fe₂O₃.
Fe : Fe₂O₃
4 : 2
0.12 : 2/4×0.12 = 0.06 mol
Mass of Fe₂O₃:
Mass = number of moles × molar mass
Mass = 0.06 mol × 159.69 g/mol
Mass = 9.58 g
An aqueous solution contains 0.20 M ammonia. One liter of this solution could be converted into a buffer by the addition of: (Assume that the volume remains constant as each substance is added.) A. 0.10 mol HNO3 B. 0.20 mol Ca(clo) C. 0.10 mol Ca(OH)2
D. 0.21 mol NH4CIO4 E. 0.21 mol HNO3
Answers
To convert the aqueous solution of 0.20 M ammonia into a buffer, we need to add a weak acid or weak base along with its conjugate acid/base pair. Among the given options, only option D, 0.21 mol NH4CIO4, contains a weak acid (HClO4) and its conjugate base (ClO4-).
Therefore, we can add 0.21 mol of NH4CIO4 to the solution to make a buffer.
Option A, 0.10 mol HNO3, is a strong acid and will completely react with ammonia, leaving no buffer solution. Option B, 0.20 mol Ca(clo), is a salt and will not provide any acid or base to form a buffer. Option C, 0.10 mol Ca(OH)2, is a strong base and will completely react with ammonia, leaving no buffer solution. Option E, 0.21 mol HNO3, is also a strong acid and will not form a buffer solution.
In summary, to convert the 0.20 M aqueous solution of ammonia into a buffer solution, we can add 0.21 mol of NH4CIO4, which contains a weak acid and its conjugate base. This will create a buffer solution that can resist changes in pH when small amounts of acid or base are added to it.
To know more about ammonia visit:
https://brainly.com/question/29519032
#SPJ11
Find volume of 2.25mol of he at stp
Answers
Explanation:
_______________________

Help me out please an thank you

Answers
what type of product results when 3-pentanone reacts with dimethylamine? o enol * amide * enolate * enamine * imine
Answers
The type of product that results when 3-pentanone reacts with dimethylamine is an enamine.
3-pentanone is a ketone with the chemical formula C₅H₁₀O, and dimethylamine is a secondary amine with the chemical formula (CH₃)₂NH. When these two compounds react, a nucleophilic addition reaction occurs.
In the reaction, the lone pair of electrons on the nitrogen atom of dimethylamine attacks the carbon atom of the carbonyl group in 3-pentanone. This results in the formation of a new bond between the nitrogen and carbon atoms, while the oxygen atom of the carbonyl group becomes protonated.
The product formed is called an enamine. An enamine is a compound that contains a double bond between a carbon and a nitrogen atom. In this case, the resulting enamine will have a methyl group (CH₃) attached to the nitrogen atom and a pentyl group (C₅H₁₁) attached to the carbon atom of the double bond.
Therefore, when 3-pentanone reacts with dimethylamine, an enamine is produced as the type of product.
To know more about enamine refer here:
https://brainly.com/question/32480395#
#SPJ11
Vhat are some of the benefits and drawbacks of recycling?
Answers
Answer:
benefits: can help reduce waste in land fills, and promotes reusability (turned into different products)
drawbacks: can be much more expensive to salvage than to just throw it in a landfill, and most of the time products cannot be recycled effectively due to its material.
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
Hydrogen sulfide, H2S, is a contaminant in natural gas. It can be removed by the reaction CH4(g) + 2H2S(g) CS2(g) + 4H2(g). Heat is required to make the reaction occur. Use this reaction to answer the following questions What would happen to the equilibrium position if the temperature were increased
Answers
Answer:
If the temperature of the system is increased, then the equilibrium position would shift to the right side. According to Le Chatelier's principle, when a stress is applied to a system in equilibrium, the system will adjust itself to counteract the stress. In this case, increasing the temperature would be an external stress on the system, and the reaction would shift to consume more of the reactants, namely CH4 and H2S, to create more of the products, CS2 and H2, thus shifting the equilibrium position towards the products.
Explanation:
Classify the type of reaction between aluminum solid and oxygen gas.
Answers
Fill The Blank! there are _____ specific amino acids required in the diet daily
Answers
There are nine specific amino acids required in the diet daily.
The building blocks of proteins, known as amino acids, are crucial for the proper operation of cells in living things. Twenty different types of amino acids, each with a distinct chemical structure and side chain, are frequently present in proteins. Nine of these 20 amino acids are regarded as essential amino acids, which means that the body cannot produce them and that they must be received from food. Together with histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine, these amino acids are considered to be essential. Reduced immunological function, muscle atrophy, and poor growth and development are just a few health issues that can arise from inadequate intake of these critical amino acids.
Learn more about amino acids here:
https://brainly.com/question/13943912
#SPJ4
Give a structural formula for the carbocation intermediate that leads to the major product in the reaction of hydrogen chloride with cis−2−butene.
Answers
The major product formed when hydrogen chloride reacts with 2−methyl−2−butene proceeds through a tertiary carbo cation intermediate.
An addition reaction occurs when 2 methyl 2 butene and hydrogen chloride are combined. The Markovnickov rule states that the carbon atom with the fewest hydrogen atoms receives the negative part of the addition. As a result, carbon 2 will be increased by the addendum's negative portion.
The carbocation will be carbon-2 as indicated in the figure that is linked to this answer since this reaction proceeds through an ionic mechanism. This carbon atom is the one to which chlorine will finally bond.
A chemical compound known as hydrogen chloride (HCl) contains a polar covalent bond between chlorine and hydrogen atoms.
Learn more about hydrogen chloride:
https://brainly.com/question/27548395
#SPJ4
1. 2Na + 2HCl --> 2NaCl + H2
2. 2KClO3 --> 2KClO2 + 3O2
3. CH4 + 3O2 --> CO2 + 2H2O
Which is a decomposition reaction?
Answers
Answer:
Explanation:
Zn + 2HCI ZnCl2 + H2
How many moles of hydrogen are produced from the reaction of 3.0 moles
of zinc?
Answers
Answer:
3 moles H₂
Explanation:
The reaction equation Zn + 2HCl => ZnCl₂ + H₂ shows a 1:1 mole ratio between Zn and molecular hydrogen (H₁). Therefore, the moles of Zn consumed in the reaction is then equal to the moles of H₂ produced. In this case using 3 moles Zn => 3 moles H₂ will be produced.
Answer:
3 moles
Explanation:
Use the ratio
Yeah, I agree with the other person
what is relative abundance isotopes
Answers
The relative abundance of isotopes is the number of atoms of a particular isotope divide by the total number of atoms of all isotopes of that element, multiplied 100 percent.
What is relative abundance isotopes?The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.
Also relative abundances refers to the relative proportions of the stable isotopes of each element. They are most often quoted as atom percentages
To calculate the percent abundance of each isotope in a sample of an element, the number of atoms of a particular isotope is usually divide by the total number of atoms of all isotopes of that element and then multiply the result by 100 since it is expressed in percentage.
Mathematically, the formula for relative abundance is given as;
R.A = ( number of atoms of isotope / total number of atoms ) x 100%
Learn more about relative abundance here: https://brainly.com/question/6844925
#SPJ1
what volume of 8.25 m naoh solution must be diluted to prepare 2.40 l of 0.500 m naoh solution?
Answers
To prepare 2.40 L of 0.500 M NaOH solution, you must dilute 145 mL of 8.25 M NaOH solution.
To prepare 2.40 L of 0.500 M NaOH solution, you need to dilute a given volume of 8.25 M NaOH solution. To find the required volume, use the dilution formula: M1V1 = M2V2, where M1 and V1 are the molarity and volume of the concentrated solution, and M2 and V2 are the molarity and volume of the diluted solution.
In this case, M1 = 8.25 M, M2 = 0.500 M, and V2 = 2.40 L. You need to find V1. Rearrange the formula to get V1 = (M2V2) / M1.
Substitute the known values into the formula: V1 = (0.500 M × 2.40 L) / 8.25 M. Calculate the result: V1 ≈ 0.145 L, or 145 mL.
You can learn more about molarity at: brainly.com/question/8732513
#SPJ11
What is chemical potential energy?
A. Energy stored by atoms
B. Energy of motion
C. Energy stored in height differences
D. Energy from gravity
Answers
Answer:
the answer is A.
i think:/
Explanation:
a sample of blood is found to contain 64.5 micrograms of valproic acid. how many milligrams of valproic acid does this blood sample contain?
Answers
The valproic acid contains 0.0645 milligrams
Conversion scale1000 microgram = 1 milligram
Data obatined from the questionFrom the question given, the following data were obtained:
Mass (in micrograms) = 64.5 microgramsMass (in milligrams) =?How to convert 64.5 micrograms to milligramsWe can convert 64.5 micrograms to milligrams as illustrated below:
1000 microgram = 1 milligram
Therefore,
64.5 micrograms = (64.5 micrograms × 1 milligram) / 1000 microgram
64.5 micrograms = 0.0645 milligrams
Thus, the valproic acid contains 0.0645 milligrams
Learn more about conversion:
https://brainly.com/question/1444594
#SPJ1
Think about multiplying each speed by a factor to calculate kinetic energy at that speed. Is there a common factor that works for every speed? If so, what’s this factor?
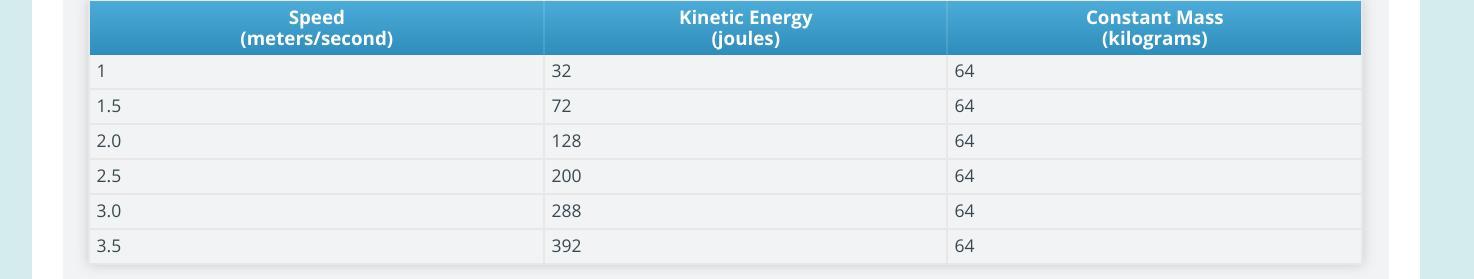
Answers
Answer:
No, there’s no common factor that works for every speed.
Explanation:
why is OH on the outside of the lewis structure for methanol?
Answers
In the Lewis structure of methanol (CH3OH), the OH group is placed on the outside because it is an important functional group that influences the chemical properties and reactivity of the molecule.
The Lewis structure is a representation of a molecule that shows the arrangement of atoms and valence electrons. In methanol, carbon (C) is the central atom bonded to three hydrogen (H) atoms and one oxygen (O) atom. The oxygen atom forms a single bond with carbon and also has two lone pairs of electrons.
The placement of the OH group (hydroxyl group) on the outside of the Lewis structure is significant because it determines the chemical behavior of methanol. The OH group consists of an oxygen atom bonded to a hydrogen atom and represents the presence of an alcohol functional group.
In organic chemistry, functional groups are specific arrangements of atoms within a molecule that give rise to characteristic chemical reactions and properties. The presence and position of functional groups can greatly influence the behavior and reactivity of a compound. In the case of methanol, the hydroxyl group provides the molecule with its characteristic properties.
know more about valence electrons here:
https://brainly.com/question/371590
#SPJ8
A rock's density is 5 G / cm3 determine the mass of the rock if it has a volume of 20cm3
Answers
Answer:
100 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volume
From the question
density = 5 g/cm³
volume = 20 cm³
We have
mass = 5 × 20 = 100
We have the final answer as
100 gHope this helps you
In the process of oxidizing i− to i2 , so42− is reduced to so2. How many moles of so2 are produced in the formation of one mole of i2 ?.
Answers
1 mole of So2 is produced in the formation of 1 mole of I2.
Oxidation and reduction is based on the addition or removal of oxygen or hydrogen atoms.so in the terms of oxygen and hydrogen the oxidation and reduction can be defined. The addition of oxygen to substances is called oxidation or the removal of hydrogen from a substance is also called oxidation. similarly, addition of hydrogen to a substance is called reduction or the removal of oxygen from a substance is called reduction.
Here in the process of oxidizing i- to i2,so42- is reduced to so2.so the required reaction becomes,
2I-(aq) +So42- (aq) +4H+ --> I2(s) +So2(g) +2H2O
From the reaction it can be seen that 1 mole of So2 is produced in the formation of 1 mole of I2 .
To learn more about Oxidation and Reduction please visit:
https://brainly.com/question/8493642
#SPJ4
A container of oxygen with a volume of 60 L is heated from 300 K to 400 k, What is the new volume?
Answers
Answer:
80L
Explanation:
V1/T1 = V2/T2
V2 = V1 T2/T1
T1 = 300K
V1 = 60L
T2 = 400K
V2 = ?
V2 = V1 T2/T1
V2 = (60L)(400K) / (300K)
V2 = 80L