Answers
Answer:
Maybe A
Explanation:
The pH of the aqueous solution is 8 means solution is basic.
How do we calculate pH?pH of any solution will be calculated by using the below equation:
pH = -log[H⁺]
Given that concentration of H⁺ ions = 1.0×10⁻⁸ M
On putting this value in the above equation, we get
pH = -log( 1.0×10⁻⁸ )
pH = -(0-8) = 8
Means the given aqueous soution is basic in nature.
Hence pH is 8 means solution is basic.
To know more about pH, visit the below link:
https://brainly.com/question/26424076
#SPJ2
Related Questions
Bohr's model was correct in assigning energy levels to electrons.
Answers
Answer:
yes that is true........
Images of the constellation Orion made using a telescope are what A. Myth. B. Science C. Pseudoscience
Answers
The Orion constellation which can be seen all around the world is positioned upon its celestial equator and it is among the noticeable and distinctive constellations in the sky. It is thought to be a Greek Mythology named after a hunter.
The Orion Nebula forms from dust, hydrogen as well as other ionized gases.
The tale and myth of Orion have various variations and explanations but one of the most popular ones is that Orion declared himself as the topmost and strongest hunter in the world.
Therefore, we can conclude that the constellation Orion made using a telescope are Myth.
Learn more about Orion Constellation here:
https://brainly.com/question/13387637?referrer=searchResults

How is steal wool and oxygen making iron oxide a chemical reaction? Why?
Answers
Answer:
When iron wool combusts, it reacts with oxygen from the air to form iron oxide. Iron oxide is a solid, so the oxygen atoms from the air add to the mass on the balance. The balance tips as the iron wool reacts with the oxygen to form solid iron oxide. from.
Explanation:
Part A
Which claim is supported by evidence in the passage?
Lewis's father was strict.
Lewis's family's troupe was successful.
Lewis was too young to remember his lines.
Lewis did not want to perform because patrons threw eggs.
Answers
Hamilton explained the choice on the On Purpose show, admitting that the pair's work connection from karting to F1 had placed a burden on an otherwise strong friendship.
Why did Lewis have a falling out with his father?Lewis Hamilton now has a close connection with his father Anthony, but this was not always the case during his Formula One tenure. Anthony had a significant influence on his son's career, notably working multiple jobs to finance Hamilton's early foray into racing until he was signed by McLaren at the age of 11.
However, his earnings outside of Formula One rose from $8 million in 2021 to $12 million in 2022. Hamilton was the highest-paid Formula One racer. He has profitable agreements with companies such as Monster energy drinks.
Learn more about Hamilton
https://brainly.com/question/31006348
#SPJ1
what is the iupac name of the following compound 4 tert butyl 3 chlorophenol 4 tert butyl 5 chlorophenol
Answers
the IUPAC name of 4 tert butyl 3 chlorophenol will be given as
3-chloro-4-(1,1-dimethylethyl)phenol.
And the structure for the compound can be given as shown in figure.
IUPAC (International Union of Pure and Applied Chemistry), serves the international scientific endeavor in the dual function of a fundamental science and mission-oriented Union.
Naming according to IUPAC is based on naming a molecule's longest chain of carbons connected via single bonds forming alkane, whether in a continuous chain or in a ring.
All other deviations, either multiple bonds or atoms other than carbon and hydrogen, are indicated by prefixes or suffixes according to a specific set of priorities.
To know more about IUPAC:
brainly.com/question/16631447
#SPJ4

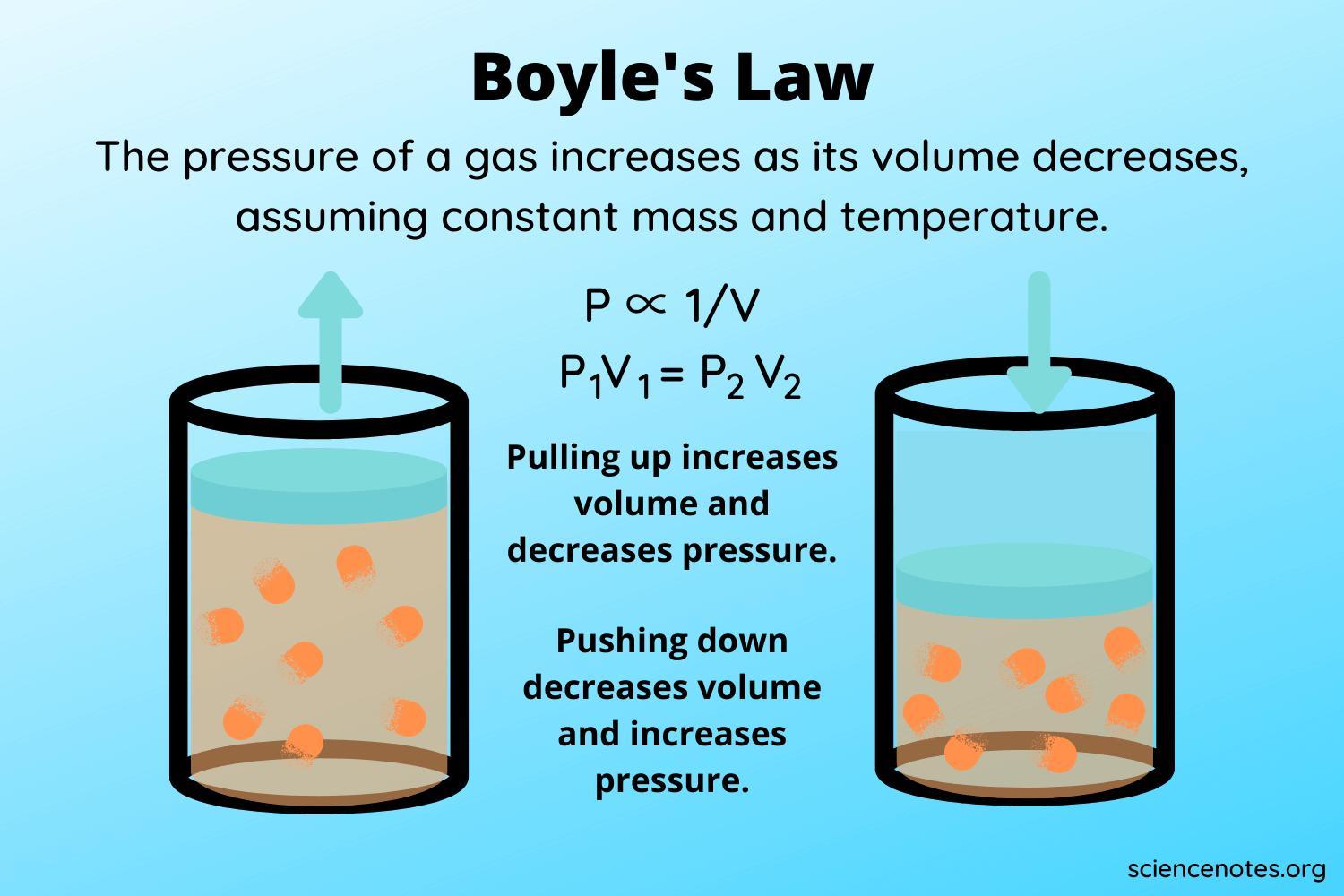
9. Which of the following gas laws is calculated with the pressure and
volume variables at a constant temperature?
Formula
4 points
P₁V₁ = P₂V₂
P₁ = first pressure
P2 = second pressure
V₁ = first volume
Answers
The gas law that is calculated with the pressure and volume variables at a constant temperature is Boyle's Law. Boyle's Law states that the pressure (P) of a gas is inversely proportional to its volume (V) when temperature (T) is held constant.
Mathematically, it is expressed as P₁V₁ = P₂V₂, where P₁ and V₁ represent the initial pressure and volume, and P₂ and V₂ represent the final pressure and volume.According to Boyle's Law, if the volume of a gas is reduced while keeping the temperature constant, the pressure will increase proportionally.
Similarly, if the volume is increased, the pressure will decrease. This relationship holds as long as the temperature remains constant throughout the process. Boyle's Law is one of the fundamental gas laws and provides insights into the behavior of gases under changing pressure and volume conditions at a constant temperature.
For more such questions on gas law
https://brainly.com/question/30233942
#SPJ11
An empty balloon sits 10 meters away from a golf ball. Jamie wants to increase the
gravitational force between the two objects by filling the balloon with a substance. Which
of the following substances will most likely increase the gravitational force between the
balloon and the golf ball?
An empty balloon sits 10 meters away from a golf ball. Jamie wants to increase the
gravitational force between the two objects by filling the balloon with a substance. Which
of the following substances will most likely increase the gravitational force between the
balloon and the golf ball?
water
cotton
air
lead pieces
Answers
To increase the gravitational force between the balloon and the golf ball, It should be filled with lead pieces. Option D
What should be done?A substance's density, which measures its mass in relation to its volume, determines how much gravitational force it produces.
Lead bits are one of the suggested materials, and they are the one that would most likely boost the gravitational force. The density of lead is much higher than that of the other listed materials.
The high density of lead will result in an increase in the gravitational pull between the balloon and the golf ball if Jamie fills the balloon with lead bits.
Learn more about density:https://brainly.com/question/29775886
#SPJ1
A sample of gas at 100 K and exerts a pressure of
5 atm in a rigid container. To what temperature
must the gas be heated in order to increase the
pressure to 8 atm?
Answers
Answer: The correct answer for the question would need to be 160 k
Explanation: I used the answer that was posted and it was inincorrect so I solved it myself to get the correct one
Determine the volume (in mL) of 1.00 M NaOH that must be added to 250 mL of 0.50 M CH3CO₂H to produce a buffer with a pH of 4.50.
Answers
Approximately 70.57 mL of 1.00 M NaOH should be added to 250 mL of 0.50 M CH3CO₂H to produce a buffer with a pH of 4.50.
To determine the volume of 1.00 M NaOH required to produce a buffer with a pH of 4.50 when added to 250 mL of 0.50 M CH3CO₂H, we need to consider the Henderson-Hasselbalch equation and the stoichiometry of the reaction.
The Henderson-Hasselbalch equation for a buffer solution is given as:
pH = pKa + log([A-]/[HA])
In this case, CH3CO₂H (acetic acid) acts as the weak acid (HA) and CH3COO- (acetate ion) acts as its conjugate base (A-). We are given that the desired pH is 4.50, and we can determine the pKa value for acetic acid from reference sources, which is approximately 4.75.
Using the Henderson-Hasselbalch equation, we can rearrange it to solve for the ratio [A-]/[HA]:
[A-]/[HA] = 10^(pH - pKa)
[A-]/[HA] = 10^(4.50 - 4.75) = 10^(-0.25) = 0.5623
This means that the ratio of the acetate ion to acetic acid in the buffer solution should be approximately 0.5623.
To calculate the required volume of NaOH, we need to consider the stoichiometry of the reaction. Acetic acid reacts with hydroxide ions (OH-) to form acetate ions and water:
CH3CO₂H + OH- → CH3COO- + H2O
The stoichiometric ratio between acetic acid and hydroxide ions is 1:1. Therefore, the volume of 1.00 M NaOH needed can be calculated using the equation:
Volume (NaOH) × 1.00 M = Volume (CH3CO₂H) × 0.50 M × 0.5623
Volume (NaOH) = (Volume (CH3CO₂H) × 0.50 M × 0.5623) / 1.00 M
Volume (NaOH) = (250 mL × 0.50 M × 0.5623) / 1.00 M
Volume (NaOH) ≈ 70.57 mL
For more such questions on buffer visit:
https://brainly.com/question/13076037
#SPJ8
Which of the following test tubes
(5 Points) would have the fastest rate of reaction? Defend your answer.
(5 Points) would have the slowest rate of reaction? Defend your answer.After presenting a reason and evidence in an argumentative essay, a writer should present
Answers
Answer:
could you explain your question more
Explanation:
URGENT!! WILL MARK ANYONE WITH ALL ANSWERS AS BRAINLIEST!!!




Answers
Answer:
1) 9 moles
2) 8.75 moles
3) 1.76 moles
4) 10.2 moles
Explanation:
Okay so mole ratio is 2:1
So, 9 moles of HI is required for 4.5 moles of Iodine gas
Mol ratio of water to CaCl2 is 2:1
So, 17.5 moles of water produced is (17.5/2) moles of CaCl2 i.e. 8.75 moles
Okay so mol ratio of Hydrogen to NH3 is 3:2
So, 2.64 moles of hydrogen is (2.64 * 2)/3 moles of NH3 i.e. 1.76 moles
Once again, mol ratio of Hydrogen to NH3 is 3:2
When 15.3 moles of hydrogen is used, (15.3 * 2)/3 moles of NH3 i.e. 10.2 moles
Hope this helps and be sure to mark this as brainliest! :)
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
help heating curve iron
at what temperature does the substance begins to boil
at what temperature does a substance begin to melt
at what temperature is a substance for a liquid and a gas
at what temperature is the substance both a solid and a liquid

Answers
At 2861 degree Celsius the iron begins to boil. At 1,538 °C the substance begins to melt.
The melting point is the point at which the liquid and solid forms of a solid can exist in equilibrium. It can also be defined as the point at which a solid changes into a liquid under normal atmospheric pressure.
The equilibrium point at which water vapor, liquid water, and solid ice can exist in equilibrium is the only point at which the pressure and temperature of water vapor are the same. The equilibrium point of water vapor is the point at which the partial vapor pressure is the same as that of liquid water at the exact temperature of 273.1600 K.
To learn more about iron, refer to the link:
https://brainly.com/question/31984794
#SPJ1
Please help with this one

Answers
Answer:
15g
Explanation:
hope this helped
This is called what ??

Answers
Answer:
Its called reflection
I need help figuring it out the answers were wrong I put in

Answers
Which elements would form metallic bonds?
Select all correct answers.
iridium
cadmium
iron
chlorine
Answers
The elements that would form metallic bonds are as follows:
IronIridiumWhat is a metallic bond?A metallic bond in chemistry is a chemical bond in which mobile electrons are shared over many nuclei and therefore, leads to electrical conduction.
Metallic bonds are formed strictly by metallic elements in the periodic table.
Iridium is a chemical element with symbol Ir with an atomic number of 77. It is a very hard, brittle, silvery-white transition metal.
Iron is also a metallic chemical element with symbol Fe.
Therefore, the elements that would form metallic bonds are as follows:
IronIridiumLearn more about metals at: https://brainly.com/question/16026653
#SPJ1
Answer:
Iridium
Cadmium
Iron
Explanation:
Test.
How many grams of sodium chloride are in 100 mL of 1.25 M sodium chloride?
Answers
Answer:
7.3125grams
Explanation:
Molarity is the measure of the molar concentration of a solution. It can be calculated using the formula as follows:
Molarity = number of moles (n) ÷ volume (V)
Based on the information given,
Molarity = 1.25 M
Volume = 100 mL = 100/1000 = 0.1 L
Molarity = n/V
1.25 = n/0.1
n = 1.25 × 0.1
n = 0.125mol
Using the below formula to calculate the mass of NaCl;
mole = mass/molar mass
Molar mass of NaCl = 23 + 35.5 = 58.5g/mol
0.125 = mass/58.5
mass = 0.125 × 58.5
mass = 7.3125 grams
m Titration Calculations: It was found that 15 cm^3 of potassium hydroxide neutralises 0,375 litres of sulfurous acid Calculate the concentration of the sulfurous acid.
Answers
Answer:49 g/L is the concentration of the acid
Explanation: simply write an equation for the reaction.
Breathing is one of the most universal and habitual practices that humans do. Most of the time, we don't even think about it. Our lungs allow us to breathe in air and provide much needed oxygen to the rest of the body. It also eliminates carbon dioxide to get rid of this waste material of our body. This breathing process is made possible because of Boyle's law.
In at most 100- word essay, explain how Boyle's law applies to human breathing apparatus and suggest ways on how to take good care of our healthy lungs.
Answers
Answer:
lets Do it dude
We can breathe air in and out of our lungs because of Boyle’s law. According to Boyle’s law, if a given amount of gas has a constant temperature, increasing its volume decreases its pressure, and vice-versa.When you inhale, muscles increase the size of your thoracic (chest) cavity and expand your lungs. This increases their volume, so pressure inside the lungs decreases. As a result, outside air rushes into the lungs. That’s because a gas always flows from an area of higher to lower pressure.When you exhale, muscles decrease the size of your chest cavity and squeeze your lungs. This decreases their volume, so pressure inside the lungs increases. As a result, air rushes out of the lungs, flowing from an area of higher to lower pressure.Explanation:
Checkmate
How many moles of Sb,03 will be formed when you have 20.0 moles of oxygen gases?
Answers
20.0 moles of oxygen react with Antimony to form 13.3 moles of Antimony (III) Oxide. We want to calculate how many moles of Antimony (III) Oxide will be formed from 20.0 moles of oxygen. This is a stoichiometry problem.
What is stoichiometry?The link between the proportional amounts of components participating in a reaction or generating a compound is known as stoichiometry, and it is often expressed as a ratio of whole integers.
Assuming a balanced chemical equation, the stoichiometric ratio between Antimony (III) Oxide and oxygen can be used to determine the number of moles of Antimony (III) Oxide formed.
For example, the balanced equation for the reaction of Antimony with O2 to form Antimony (III) Oxide is:
4 Antimony + 3 O2 → 2 Antimony (III) Oxide
From this equation, it can be seen that 3 moles of oxygen react with 2 moles of Antimony (III) Oxide . Therefore, if there are 20.0 moles of O2, then the number of moles of Antimony (III) Oxide formed would be:
20.0 moles oxygen × (2 moles Antimony (III) Oxide / 3 moles oxygen) = 13.3 moles Antimony (III) Oxide.
To know more about moles visit:-
https://brainly.com/question/26416088
#SPJ1
imple of argon gas at standard pressure occupies 1492 mL. At constantperature, what volume does the gas occupy if the pressure increases to 768Hg?

Answers
What we're going to do, is to use Boyle's law:
In this problem, we're given the following information:
P1 = 760mmHg (Which is the standard pressure)
V1 = 1492 mL
P2 = 768 mmHg
V2 = ?
Let's replace these values in the equation and then solve for V2:
The gas will occupy a volume about 1480 ml.

What is SO2 shape name?
Answers
Answer:Molecular Formula SO2
Hybridization Type sp2
Bond Angle 119o
Geometry V-Shaped or Bent
Explanation:
hope this helped <3
*Will give Brainliest* What is the average atomic mass of one molecule of methane, which has one 12C atom and four 1H atoms?
Answers
Answer:
The atomic mass of methane (CH4)is 12 amu for the carbon plus 4 x 1 amu for the four hydrogens, for a total of 16 amu. Therefore, the molar mass of methane is 16g.
2. 4.6gof X is burnt completelyto produce 6.2g of X oxide (X,O). M (0) = 16 gmol ¹. Calculate the amount of oxygen that reacted in this experiment. [2 MARKS]
[ii] calculate the mass of 1 mole of x.[2mark]
[iii] predict and give a reason explaining the reaction of x2o in water.[1mark]
Answers
As per the given data, 1.6 grams of oxygen reacted in this experiment.
To calculate the amount of oxygen that reacted in the experiment, we need to determine the difference in the mass of X oxide (X,O) formed and the mass of X initially used.
Given:
Mass of X = 4.6 g
Mass of X oxide (X,O) = 6.2 g
To find the amount of oxygen that reacted:
Mass of oxygen = Mass of X oxide - Mass of X
= 6.2 g - 4.6 g
= 1.6 g
Therefore, 1.6 grams of oxygen reacted in this experiment.
Calculate the mass of 1 mole of X:
Given that the mass of X is 4.6 g, we can calculate the molar mass of X by dividing the mass by the number of moles:
Molar mass of X = Mass of X / Number of moles of X
Molar mass of X = 4.6 g / 0.1 mol
Molar mass of X = 46 g/mol
Therefore, the mass of 1 mole of X is 46 grams.
Thus, the answer is 46 grams.
For more details regarding moles, visit:
https://brainly.com/question/30885025
#SPJ1
When all the soil pores are essentially water-filled, flow is termed _______________ .
Unsaturated
Saturated
Gravitional
Rapid
Answers
in a land ecosytem , some organisms only live in the soil under rocks logs or plants . What would be a resonable prediction about how theses organisms would be affected if humans removed the coverings .
Answers
Answer:
The number of these organisms in the soil would decrease.
Explanation:
Explain how this change will impact the transfer of thermal energy in the solar cooker design and describe one possible design improvement the students can make to compensate for this change
Answers
Answer:it conduct thermal conductivity because of the heat it holds
Explanation:
When energy is transformed from one form to another, the appliance could be redesigned in such a way that this transfer is minimized.
Transfer of thermal energyWe know from the principle of transfer of energy that energy can neither be created nor destroyed but is transformed from one form to another. The image is not shown here but I will try to explain what energy transfer is.
Usually, when energy is transformed from one form to another, the appliance could be redesigned in such a way that this transfer is minimized.
Learn more about energy transfer:https://brainly.com/question/18649915?
#SPJ2
PLS HELPPP ITS DUE VRY SOON

Answers
In a molecule or covalent bonding, elements having Octet electrons to obtain the stability with eight electrons.
One element that does not follow the octet rule is hydrogen. For stability only require stable electrons.
A molecule can present covalent bond but can be chemical.
What is a covalent bond?Covalent bonding is a type of chemical bonding that occurs when two or more atoms share electrons in order to form a strong bond between them. This type of bond is formed when atoms share electrons in a way that gives each atom a full outer shell of electrons, known as the octet rule. This means that all atoms in the bond must have a total of eight electrons in their outermost shell. The atoms involved in a covalent bond form a molecule, which is held together by the shared electrons.
The octet rule is a guideline that states that atoms will bond in a way that allows them to have a full outer shell of electrons. This means that they must share electrons in order to reach a total of eight electrons in their outermost shell. The sharing of electrons creates a strong bond between the atoms that form a molecule. The sharing of electrons also results in the creation of a covalent bond.
However, not all elements follow the octet rule. For example, hydrogen only requires two electrons to reach a stable state, so it does not need to share electrons in order to reach the octet rule. Therefore, a hydrogen molecule will not have a covalent bond.
To know more about covalent bonds, visit
brainly.com/question/19382448
#SPJ1
What can you deduce about the molecular composition of the reactants in a chemical reaction with the following atomic masses?
Reactants: (12 + 1 + 1 + 1 + 1) + (24 +16) = 56 u
Answers
Stoichiometric amounts relate to the proportional amounts of reactants and products in a balanced chemical equation.
What transpires during a chemical reaction to the molecules of the reactants?
Only atoms from the reactants can wind up in the products of a chemical reaction. No atoms are annihilated or made into new ones. To create the products, the reactants come into contact with one another, the bonds between their atoms are broken, and the atoms then rearrange and establish new bonds.
The frequency of collisions between the two reactants will grow as the reactant concentration rises. There are times when collisions don't cause a response (atoms misaligned or insufficient energy, etc.). More collisions and reaction possibilities result from higher concentrations.
To learn more about atoms use:
https://brainly.com/question/6258301
#SPJ1