2. How many moles of water are in 3.80 * 10 ^ 23 molecules of H2O
Answers
Answer:
0.1 moles
Explanation:
Related Questions
how much energy is needed to convert 120g of ice at -35°C to steam at 150°C?
Answers
Please the app isn’t working and I can’t find other questions that got answered

Answers
Given:
Sum of masses of two isotopes = 371.9087 u
Re-185 natural abudance = 37.40%
Re-187 natural abudance = 62.60%
Known:
atomic weight of Re = 186.207 u
Atomic mass of Re-185:
To find the atomic mass of Re-185, take the total mass given and subtract atomic weight.
abundance of Re-185 = 37.40% = 0.3740
(371.9087 - x) = atomic weight of Re-187 in u
To find mass of Re-187:
abundance of Re-187 = 62.60% = 0.6260
Solution:
Step 1. Multiply x times the abundance of Re-185 and multiply (371.9087 - x) times the abundance of Re-187.
Re-185: (0.3740)(x) = 0.3740x
Re-187: (0.6260)(371.9087 - x) = 232.8148462 - 0.6260x
Step 2. Add the results and set them equal to 186.207.
0.3740x + 232.8148462 - 0.6260x = 186.207
Step 3. Solve for x by subtracting 232.8148462 from both sides and then divide both sides by -0.2520.
0.3740x + 232.8148462 - 0.6260x - 232.8148462 = 186.207 -
232.8148462
0.3740x - 0.6260x = -46.6078462
-0.2520x = -46.6078462
-0.2520x/-0.2520x = -46.6078462/-0.2520
x = 184.9517706 u
Step 4. Atomic weights of Re-185 and Re-187.
x = 185.0 u = the atomic weight of Re-185
(371.9087 - 184.9517706) = 186.9569294 = 187.0 u = the atomic weight of Re-187
Therefore the atomic weight of Re-185 is 185.0 u, and the atomic weight of Re-187 is 187.0 u.
Can you help solve each question I answered incorrectly
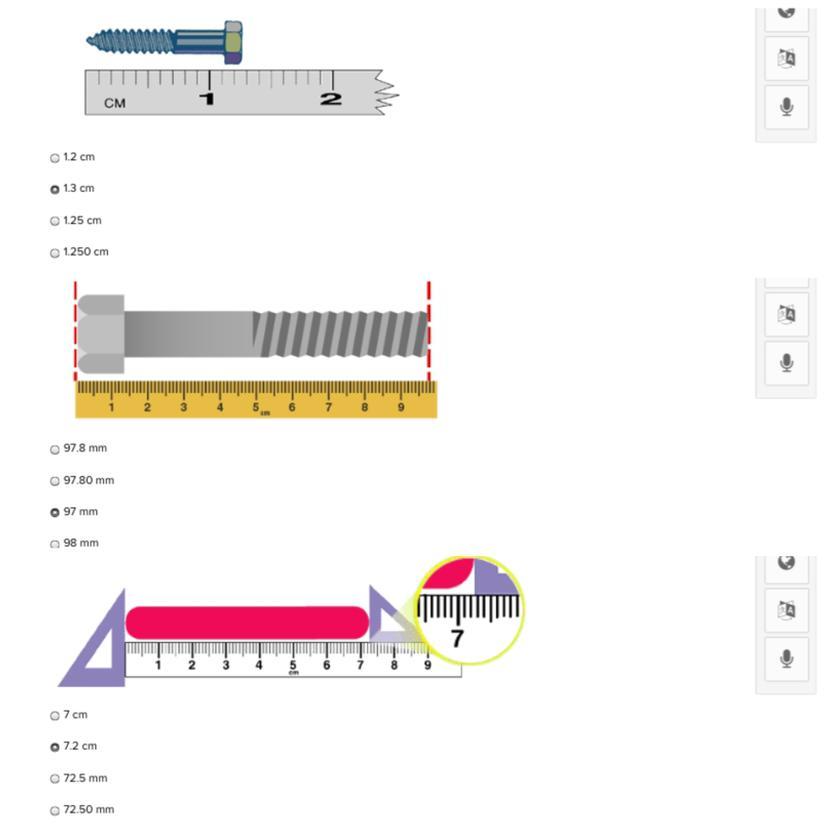
Answers
Answer:
1.2cm
97.8mm
7cm
How solve stiochiometry problem easily
Answers
Answer:
Balance the equation.Convert units of a given substance to moles.Using the mole ratio, calculate the moles of substance yielded by the reaction.Convert moles of wanted substance to desired units.
what is law of motion...........
Answers
\( \huge \boxed{\mathbb{QUESTION} \downarrow}\)
What's the law of motion ?\( \large \boxed{\mathfrak{Answer \: with \: Explanation} \downarrow}\)
In physics, there are 3 laws of motion stated by Issac Newton who was a famous mathematician & physician.
Newton's 1st Law of Motion ⇨ The 1st law states that 'any object will continue in its state of rest or motion until or unless an external unbalanced force acts on it.' It's also known as the Law of Inertia.Newton's 2nd Law of Motion ⇨ The 2nd law states that 'the acceleration of an object is directly proportional to the net force and inversely proportional to the mass of the object.' The formula for this law is 'f = ma, where, f = force, m = mass & a = acceleration.'Newton's 3rd Law of Motion ⇨ The 2rd law of motion states that 'for every action that takes place, there'll be an equal & opposite reaction'. This is perhaps the most commonly known law & the recoiling of the gun is the best example for this law of motion.Balance the following redox reaction if it occurs in basic solution. What coefficients in front of Al and Ft in the balanced reaction? Al(s) + F2(g) rightarrow Al^3+ (aq) + F^- (aq) A) Al = 2, F2 = 3 B) Al = 2, F2 = 6 C) Al = l, F2 = 1 D) Al = 2, F2 = 1E) Al = 3, F2 = 2
Answers
The balanced redox reaction in basic solution is:
\(\begin{aligned} \ce{2Al(s) + 3F2(g) + 6OH^{-}(aq) &- > 2Al(OH)3(aq) + 6F^{-}(aq)} \ \text{Coefficients: Al} &= 2, \text{ F2} = 3 \end{aligned}\)
To balance the given redox reaction in basic solution, we need to follow these steps:
Write the unbalanced equation:
\(\ce{Al(s) + F2(g) - > Al^3+(aq) + F^-(aq)}\)
Identify the oxidation states of each element:
\(\begin{aligned} &\ce{Al(s)} &&\ce{- > } &&\ce{Al^3+(aq)} \ &\text{0} && &&\text{+3} \ \ &\ce{F2(g)} &&\ce{- > } &&\ce{2F^-(aq)} \ &\text{0} && &&\text{-1} \end{aligned}\)
Determine which element is oxidized and which is reduced:
In this case, Al is oxidized (loses electrons) and F2 is reduced (gains electrons).
Balance the half-reactions:
Oxidation half-reaction:
\(\ce{Al(s) - > Al^3+(aq) + 3e-}\)
Reduction half-reaction:
\(\ce{F2(g) + 2e- - > 2F^-(aq)}\)
Balance the electrons:
Multiply the oxidation half-reaction by 2 to balance the electrons:
\(\ce{2Al(s) - > 2Al^3+(aq) + 6e-}\)
Combine the half-reactions:
Add the half-reactions together and cancel out the electrons:
\(\ce{2Al(s) + 3F2(g) - > 2Al^3+(aq) + 6F^-(aq)}\)
Balance the charges:
Add hydroxide ions (OH-) to both sides of the equation to balance the charges:
\(\ce{2Al(s) + 3F2(g) + 6OH^{-}(aq) - > 2Al(OH)3(aq) + 6F^-(aq)}\)
Check the balance:
Count the atoms of each element and make sure they are balanced on both sides of the equation.
Identify the coefficients:
The coefficients in front of Al and F2 are Al = 2, F2 = 3.
For more questions like Redox reaction click the link below:
https://brainly.com/question/13293425
#SPJ11
A sample of gas occupies 75.5 mL at -14.2°C. What volume does the sample occupy at 146.7 °C?
Answers
a restriction enzyme recognizes the sequence 5ʹ-gtcatgac-3ʹ and makes staggered cuts. which statement is most likely to be true?
Answers
The most likely true statement about a restriction enzyme recognizing the sequence 5ʹ-gtcatgac-3ʹ and making staggered cuts is: "The restriction enzyme produces fragments with sticky ends".
When a restriction enzyme recognizes a specific DNA sequence, it cuts the DNA at or near that sequence. Staggered cuts refer to cuts made at different positions on the two DNA strands, resulting in fragments with overhanging ends. These overhanging ends are often referred to as sticky ends because they can base pair with complementary sequences.
In the given sequence 5ʹ-gtcatgac-3ʹ, the restriction enzyme would recognize and cut between the G and the T bases, resulting in staggered cuts. This would produce fragments with complementary overhangs: 5ʹ-GTCATG-3ʹ and 5ʹ-GTCA-3ʹ. These overhanging ends can then bind or anneal with complementary sequences during DNA manipulation, such as in cloning or DNA ligation reactions.
Learn more about restriction enzymes at https://brainly.com/question/15278286
#SPJ11
How many moles of propane
react when 294 g of CO2 form?
C3H8 +502 → 3CO₂ + 4H₂O
Answers
2.23 moles of propane react when 294 g of CO₂ is formed .
What is moles ?Moles is a unit which is equal to the molar mass of an element.
A reaction is given
C₃H₈ +50₂ → 3CO₂ + 4H₂O
Grams of CO₂ formed = 294 gm
In moles = 294 /44 = 6.68 moles.
Let x be the moles of C₃H₈ is x
Mole ratio of CO₂ to C₃H₈ = 3 : 1
so
6.68 /x = 3/1
x = 6.68 /3 = 2.23 moles
Therefore 2.23 moles of propane react when 294 g of CO₂ is formed .
To know more about Moles
https://brainly.com/question/26416088
#SPJ1
I NEED HELP PLEASE, THANKS! :)
Combustion reactions are a notable source of carbon dioxide in the environment. Using the following balanced equation, how many grams of carbon dioxide are formed when 100.00 g of propane (C3H8) is burned? Express your answer to the correct number of significant figures.

Answers
Answer:
\(\large \boxed{\text{299.4 g}}\)
Explanation:
We will need a chemical equation with masses and molar masses, so, let's gather all the information in one place.
Mᵣ: 44.10 44.01
C₃H₈ + 5O₂ ⟶ 3CO₂ + 4H₂O
m/g: 100.00
To solve a stoichiometry problem, you must
Use the molar mass to convert mass of C₃H₈ to moles of C₃H₈ Use the molar ratio to convert moles of C₃H₈ to moles of CO₂ Use the molar mass to convert moles of CO₂ to mass of CO₂
1. Moles of C₃H₈
\(\text{Moles of C$_{3}$H}_{8} = \text{100.00 g C$_{3}$H}_{8} \times \dfrac{\text{1 mol C$_{3}$H}_{8}}{\text{44.10 g C$_{3}$H}_{8}} = \text{2.268 mol C$_{3}$H}_{8}\)
2. Moles of CO₂
The molar ratio is 3 mol CO₂:1 mol C₃H₈
\(\text{Moles of CO}_{2} = \text{2.268 mol C$_{3}$H}_{8} \times \dfrac{\text{3 mol CO}_{2}}{\text{1 mol C$_{3}$H}_{8}} = \text{6.803 mol CO}_{2}\)
3. Mass of CO₂
\(\text{Mass of CO}_{2} = \text{6.803 mol CO}_{2} \times \dfrac{\text{44.01 g CO}_{2}}{\text{1 mol CO}_{2}} = \textbf{299.4 g CO}_{2}\\\text{The mass of CO$_{2}$ required is $\large \boxed{\textbf{299.4 g}}$}\)
Answer:
Number of moles of propane:
=Mass in grams ÷ Relative molecular Mass
= 100/((12*3) + (1*8))
= 100 ÷ 44
= 2.2727
Mole ratio propane:carbon (IV) oxide = 1:3(from the equation)
Number of moles of CO2 = 3*2.2727 = 6.8181
Mass in grams = Relative molecular Mass * Number of moles
= 44 * 6.8181
= 299.9964 grams
Explanation:
how many electrons occupy a filled 6s sublevel
Answers
Answer:
2 electrons
Explanation:
every s sublevel can only be occupied by 2 electrons so a filled 6s sublevel would have 2 electrons
What change occurs during reduction?.
Answers
An oxidation-reduction reaction, often known as a redox reaction, occurs when two chemical species exchange electrons (the atoms, ions, or molecules involved in the reaction).
Redox reactions are ubiquitous; they occur during the burning of fuels, the corrosion of metals, photosynthesis, and cellular respiration, among other processes. Some species go through oxidation, which is the loss of electrons, while others go through reduction, which is the gain of electrons, during a redox process.
Redox reactions, sometimes called oxidation-reduction processes, involve the movement of electrons from one species to another. When a species receives electrons, it is said to be reduced, but when a species loses electrons, it is said to be oxidized.
To know more about reduction, please refer:
https://brainly.com/question/20758765
#SPJ4
How can I solve this using a T chart

Answers
Answer:
The answer to your question is given below
Explanation:
The balanced equation for the reaction is given below:
2KClO₃ —> 2KCl + 3O₂
From the balanced equation above,
2 moles of KClO₃ decomposed to produce 2 moles of KCl and 3 moles of O₂.
Next, we shall determine the number of mole of KClO₃ that will decompose to produce 9 moles of O₂. This can be obtained as follow:
From the balanced equation above,
2 moles of KClO₃ decomposed to produce 3 moles of O₂.
Therefore, Xmol of KClO₃ will decompose to produce 9 moles of O₂ i.e
Xmol of KClO₃ = (2 × 9)/3
Xmol of KClO₃ = 6 moles
Thus, 6 moles of KClO₃ is needed for the reaction.
Next, we shall determine the number of mole KCl that will be produced by the decomposition of 6 moles of KClO₃. This can be obtained as follow:
From the balanced equation above,
2 moles of KClO₃ decomposed to produce 2 moles of KCl.
Therefore, 6 moles of KClO₃ will also decompose to produce 6 moles of KCl.
Finally we shall represent the reaction in a chart as illustrated below:
2KClO₃ —> 2KCl + 3O₂
6 moles —> 6 moles | 9 moles
Materials
Crayon
Table 2. Investigating crayons and candle when heated
Property before
heating
Property after heating
Answers
Answer:
1 - Crayon
Before heating is solid
After heating is liquid
2 - Candle
Before heating is solid
After heating is liquid
Explanation:
Crayon when heated become liquid at a temperature of 115 degrees Fahrenheit or above. When the temperature reached to 120 degrees Fahrenheit for crayon it melts down completely.
When Candle is heated it melts and liquid form of wax falls off which again becomes solid in second after reaching the floor. The melting point of candle is 130 degree Fahrenheit or above.
30 points if you tell me your favorite food! ☺️
Answers
Answer:
Pizza, salad, tacos, burgers, etc.
Explanation:
:D
A 3. 245g sample of titanium chloride was reacted with sodium metal, producing sodium chloride and metallic titianium. After the sodium chloride was washed out, the remaining titanium metal weighed 0. 819g. What is the empirical formula of the titanium chloride
Answers
To find the empirical formula of the titanium chloride, we need to use the given information to determine the moles of titanium and chlorine in the original compound, and then use those values to find the simplest whole-number ratio of atoms in the empirical formula.
First, we can find the moles of titanium in the original compound using the mass of the titanium metal produced:
mass of titanium metal = 0.819 g
molar mass of titanium = 47.867 g/mol
moles of titanium = mass of titanium metal / molar mass of titanium
moles of titanium = 0.819 g / 47.867 g/mol
moles of titanium = 0.0171 mol
Next, we can use the law of conservation of mass to find the moles of chlorine in the original compound:
moles of chlorine = moles of titanium
Now we can find the mass of chlorine in the original compound using the moles of chlorine and the molar mass of chlorine:
moles of chlorine = 0.0171 mol
molar mass of chlorine = 35.453 g/mol
mass of chlorine = moles of chlorine x molar mass of chlorine
mass of chlorine = 0.0171 mol x 35.453 g/mol
mass of chlorine = 0.606 g
Finally, we can use the masses of titanium and chlorine to find the empirical formula of the titanium chloride. The empirical formula gives the simplest whole-number ratio of atoms in a compound, so we need to divide the masses of each element by their respective atomic masses to get the number of moles of each element:
moles of titanium = 0.0171 mol
moles of chlorine = 0.606 g / 35.453 g/mol = 0.0171 mol
The ratio of titanium to chlorine is 1:1, so the empirical formula of the titanium chloride is TiCl<sub>1</sub>, or simply TiCl.
To know more about empirical refer here
https://brainly.com/question/977538#
#SPJ11
A student measures the mass of an object and obtains three values: 21.57 g,
22.04 g, and 21.35 g. What is the average mass of the object?
Answers
Answer:
21.65
Explanation:
The average = total divided by the number of readings
21.57
22.04
21.35
Total = 64.96
Average = 64.96 / 3 = 21.65
Consider the following reaction… 5C + 2SO2 CS2 + 4CO a) How many moles of CS2 would be produced by reacting 9.50 moles of SO2 with an excess of C? _________________ b) How many grams of C would be needed to fully react 5.5 L of SO2 at STP? _________________ c) How many liters of CO can be produced from 20.0 moles of C at STP?
Answers
We first verify that the equation is balanced. We have 5 carbons (C), 2 sulfurs (S), and 4 oxygens (O) on each side of the reaction. So the reaction is balanced.
a) Now if we look at the reaction we can see that when 2 moles of SO2 react, 1 mole of CS2 is produced. That is, the ratio is 2 to 1. For each mole of SO2 half as many moles of CS2 will be produced.
So if we have 9.5 moles of SO2 we will have 9.5/2 moles, that is 4.75 moles of CS2.
Answer a) By reacting 9.50 moles of SO2 with an excess of it would be produced 4.75 moles of CS2.
Now, for the following parts of the question, we can apply the ideal gas law. This is because the reaction is in the gas phase and the law applies only to gases.
\(PV=nR_{}T\)Where,
P= Pressure at STP = 1 atm
T= Temperature at STP = 273.15K
R= Ideal law constant = 0.08206 (atm L)/(mol K)
V= Volume of the gas
n= Numer of moles
b)We clear n and we replace the known values of SO2 to find the number of moles of SO2 that react.
\(\begin{gathered} n=\frac{PV}{RT}=\frac{1at_{}m\times5.5L}{0.08206\frac{atm.L}{\text{mol}\mathrm{}K}\times273.15K} \\ n=0.24mol\text{ SO}_2 \end{gathered}\)Now, for each mole of SO2 that reacts we need 5/2 moles of C, that is 0.24x5/2=0.61 moles of C.
We use mass molar of C to calculate the grams.
Mass molar of C=12.01g/mol
Mass of C= Moles of C x Mass Molar
Mass of C= 0.61 mol x 12.01 g/mol = 7.37 g
So, To fully react 5.5 L of SO2 at STP we will need 7.37 g of C.
c)We apply the gas law again but this time we clear the volume.
We also take into account that for each mole of C, 4 moles of CO are produced, so if we have 20 moles of C we will produce 20x4=80 moles of CO.
\(\begin{gathered} V=\frac{nRT}{P} \\ V=\frac{80mol\times0.08206\frac{atm.L}{mol.K}\times273.15K}{1atm} \\ V=\text{ 1793.18 L} \end{gathered}\)So, from 20.0 moles of C at STP can be produced 1793.18 liters of CO
Water from jordan lake was analyzed for its fe3+ content. A 20. 0-ml sample of lake water was acidified with nitric acid and treated with excess kscn to form a red complex (kscn itself is colorless). The solution was then diluted to 50. 0-ml and put in a 1. 00 cm pathlength cell, where it yielded an absorbance of 0. 345. For comparison, a 5. 0-ml reference sample of 4. 80 x 10-4 m fe3+ was treated with hno3 and kscn and diluted to 50. 0 ml. The reference solution was also placed in a 1. 00-cm cell and gave an absorbance of 0. 512. What is the concentration of fe3+ in jordan lake?.
Answers
The concentration of Fe³⁺ in Jordan Lake is = 8.09 × 10⁻⁵ M
What is Lambert-Beer law?The Beer-Lambert Law, also known as Beer's Law, Lambert-Beer Law, or Beer-Lambert-Bouguer Law, relates the attenuation of light to the properties of the material through which the light passes.
According to Lambert-Beer's law, the absorbance of a sample is directly proportional to its concentration.
The reaction that produces a red complex
Fe³⁺ + KScN ⇒ Fe ( SCN )₃ ( red complex )
Concentration of Fe³⁺ in reference sample
= 4.80x10⁻⁴ × ( 5.0 / 50.0 ) = 4.80 × 10⁻⁵M
Reference sample was diluted from 5.0 mL to 50.0 mL
Concentration of 4.80 × 10⁻⁵M has an absorbance = 0.512
Given that Lake sample absorbance is 0.345
Concentration of lake sample :
= absorbance of lake sample × ( conc of reference sample / absorbance )
= 0.345 × (4.80* 10⁻⁵ / 0.512) = 3.23 × 10⁻⁵M.
Concentration of Fe³⁺ in Jordan lake
= 3.23 × 10⁻⁵ × ( 50.0mL / 20.0mL) = 8.09 × 10⁻⁵ M
Solution was diluted from 20.0 mL to 50.0 mL
Hence we can conclude that The concentration of Fe³⁺ in Jordan Lake is = 8.09 × 10⁻⁵ M.
To know more about Lambert-Beer law visit:
https://brainly.com/question/24183759
#SPJ4
Write a balanced equation for the transmutation that occurs when a nitrogen-13 nucleus emits a positron.
A.
137N→138O+0−1e
7
13
N
→
8
13
O
+
−
1
0
e
B.
137N→136C+01e
7
13
N
→
6
13
C
+
1
0
e
C.
137N→95B+42He
7
13
N
→
5
9
B
+
2
4
H
e
D.
137N→137N+00γ
7
13
N
→
7
13
N
+
0
0
γ
Answers
C is the answer. odoriferous
A change in dosage from the prescription of food and a delayed gastric emptying time are all examples of things that can alter A. AbsorptionB. DistributionC. Metabolism D. Elimination.
Answers
A. Absorption. Both processes are relatod to the way drugs are absorbed into the system.
2. Describe the changes in bonding and hybridization of the carbon atoms that take place during the polymerization of styrene to form polystyrene.
Answers
During the polymerization of styrene to form polystyrene, the bonding and hybridization of the carbon atoms undergo significant changes.
In styrene, the carbon atoms are originally sp2 hybridized and form a conjugated system of double bonds in the aromatic ring. However, during the polymerization process, the double bonds in styrene undergo addition polymerization.As a result, the carbon atoms in polystyrene undergo a transformation in hybridization from sp2 to sp3. The double bonds break, and new sigma bonds are formed between the carbon atoms, leading to the formation of a long chain of repeating units.
This change in hybridization allows the carbon atoms in polystyrene to form stronger and more stable sigma bonds with neighboring carbon atoms or other substituents. Consequently, the structure of polystyrene becomes a three-dimensional network of carbon-carbon bonds throughout the polymer chainThe transition from an aromatic, conjugated system in styrene to a saturated, three-dimensional polymer structure in polystyrene is vital in the polymerization process, providing the desired properties and structural integrity to the resulting polymer.
To know about more hybridization,sp2 hybridized,carbon-carbon bonds,sigma bonds visit:
https://brainly.com/question/29020053
https://brainly.com/question/31610604
https://brainly.com/question/29663260
https://brainly.com/question/31659836
#SPJ11
Using the formula M1V1 = M2V2 , how many milliliters of a 2. 50 M hydrochloric acid solution is required to make 100. 0 mL of a 0. 750 M solution?.
Answers
Dilution of the solution can be calculated by the formula of the molarity and volume. The initial volume of 2.50 M solution was 30 mL.
What is the relationship between molar concentration and dilution?Molar concentration or the dilution factor is in an inverse relationship and with an increase in the dilution, the molarity of the solution decreases.
Given,
Initial molarity = 2.50 M
initial volume = ?
Final molarity = 0.750 M
Final volume = 100.0 ml
Substituting values in the formula:
\(\begin{aligned}\rm V_{1} &= \rm \dfrac{M_{2}V_{2}}{M_{1}}\\\\&= \dfrac{0.750 \times 100}{2.50}\\\\&= 30 \;\rm mL\end{aligned}\)
Therefore, 30 mL was the initial volume of the solution before it was diluted.
Learn more about dilution here:
https://brainly.com/question/26896011
Find the pOH for the following:
A 1.34 x 10^-4 M solution of hydrochloric acid.
A) 10.13
B) 3.87
C) 4
D) 10

Answers
Answer:
A) 10.13 is the answer
Explanation:
Since HCl is a strong acid, its pH can be found by simply plugging it into the equation:
pH = -log[H+]
*H+ is just the concentration you were given*
Now that you have the pH, you just subtract it from 14 to find the pOH
I have attatched my work below. Hope this helps! :^)

To find the pOH of a solution, you can use the formula: pOH = -log10[OH-]
What is pOH?pOH is a measure of the concentration of hydroxide ions (OH-) in a solution. It is the negative logarithm (base 10) of the hydroxide ion concentration.
In this case, since we have hydrochloric acid (HCl), we need to consider the concentration of hydroxide ions (OH-) produced when HCl dissociates in water.
HCl is a strong acid and completely dissociates, meaning it produces an equal concentration of hydrogen ions (H+) and chloride ions (Cl-) in water. Since there are no hydroxide ions produced, the concentration of hydroxide ions is 0.
Therefore, the pOH of a hydrochloric acid solution is 0, and none of the options provided (A, B, C, D) is the correct answer.
Learn more about pOH at:
https://brainly.com/question/17144456
#SPJ2
which scientist concluded that all cells came from existing cells?
Please help!!
Answers
Answer:
Theodor Schwann
Explanation:
The classical cell theory was proposed by Theodor Schwann in 1839. There are three parts to this theory
40 points/ brainliest
the first step in planning effective writing is to consider the _____.
main idea
audience
style
purpose
Answers
The first step in planning effective writing is to consider the main idea.
Considering the main idea or topic that will be discussed will help the writer to stay focused and organized throughout the writing process.
Next, the writer needs to consider the audience that will be reading their writing. Knowing who the audience is will help the writer to tailor their writing style and language to better connect with the readers.
Style is another important aspect to consider when planning writing. The style of writing can greatly impact how the message is received by the audience. The writer must choose the appropriate tone, structure, and language to convey their message effectively.
Finally, the writer needs to consider the purpose of their writing. This will help them to determine the goals and objectives of their writing and how to structure the content to achieve those goals.
In conclusion, by considering the main idea, audience, style, and purpose, the writer can better plan and execute effective writing that effectively conveys the intended message to the reader. This is why these elements are crucial when it comes to planning effective writing.
Learn more about writing here: https://brainly.com/question/31119902
#SPJ11
What are the six common types of radioactive decay? What condition usually leads to each type of decay? (Select all that apply.) Gamma emission: The nucleus is in an excited state. Alpha emission: Z> 83. Beta emission: N/Z is too small. Positron emission: N/Z is too large. Spontaneous fission: Mass number<89. Gamma emission: The nucleus is in a ground state. Alpha emission: 2 <83. Delta emission: The nucleus is in a ground state. Delta emission: The nucleus is in an excited state. Positron emission: N/Z is too small. Positron capture: N/Z is too small. Positron capture: N/Z is too large. Electron capture: N/Z is too small. Spontaneous fission: Mass number > 89, Electron capture: N/Z is too large. Beta emission: N/Z is too large.
Answers
There are six common types of radioactive decay: alpha emission, beta emission, gamma emission, positron emission, electron capture, and spontaneous fission.
Each type of decay is usually caused by specific conditions. Alpha emission occurs when the nucleus has a high atomic number (Z> 83) and too many protons for stability. Beta emission happens when the ratio of neutrons to protons (N/Z) is too small or too large. Gamma emission occurs when the nucleus is in an excited state or ground state. Positron emission is usually caused by N/Z being too small or too large, and positron capture occurs when N/Z is too small or large. Electron capture occurs when N/Z is too small or too large. Finally, spontaneous fission occurs when the nucleus has a mass number greater than 89. These different types of decay are important to understand in order to fully grasp the complex process of radioactivity and its effects.
To know more about fission visit:
https://brainly.com/question/82412
#SPJ11
Loneliness
Harsh weather
Hard, dry ground
The items on this list helped convince —

Answers
What was the purpose of rinsing with hexanes in the Cyalume synthesis procedure? What was the purpose of rinsing with water in the Cyalume synthesis procedure? Why is it important/useful to prepare active ingredients that contain an amino group as ammonium chloride salt? Draw (chemdraw) the whole mechanism of bupropion HCl synthesis
Answers
In this course of union of Cyalume, the motivation behind flushing with hexane" is the filtration of Response mass for precipitate take the strong and give washing with more.
Hexane and their reaction to the filter Mass and to acquire more pure products. This product was not dissolved in the solvent hexane, resulting in a brown color and product.
b) In this Synthesis of Cyalume 1 process, the purpose of rinsing with water is to wash the product with water and filter it before washing it with hexane to make a slurry and get a brown color precipitate.
Ammonium chloride is a white glasslike strong. Water dissolves it (37%). The environmental threat is the primary danger. It is necessary to take immediate measures to stop its spread to the environment. It is utilized to make other ammonium compounds, as a welding motion, as a manure, and for the vast majority different purposes.Ammonium chloride salt :Ammonium chloride is a salt that makes the body and the urine more acidic. Ammonium chloride keeps up with pH and applies a gentle diuretic impact. This acid-forming salt is used to treat coughs because it also has an expectorant effect by irritating the mucous membranes.
Ammonium is the counterion in ammonium chloride, an inorganic chloride. It plays a role as an inhibitor of ferroptosis. It is an inorganic chloride and an ammonium salt.
Ammonium chloride can be found in cleanser, hair tone and fade, body wash and cleaning agent, facial chemical, conditioner, hand dishwashing cleanser, as well as in shower oils and salts. Because it is an ionic compound, ammonium chloride is utilized as an important electrolyte in dry cell batteries.
Learn more about ammonium chloride salt :
brainly.com/question/12969993
#SPJ4
In the Cyalume synthesis procedure, rinsing with hexanes is performed to remove impurities and unreacted starting materials that are soluble in hexanes.
Hexanes is a nonpolar solvent that can effectively dissolve nonpolar compounds while leaving behind the desired product or polar impurities. By rinsing with hexanes, the impurities can be separated from the desired product, improving the purity of the final product.
Rinsing with water in the Cyalume synthesis procedure is carried out to eliminate water-soluble impurities. Water can dissolve polar compounds, including water-soluble impurities, while the desired product may remain insoluble or less soluble. Rinsing with water aids in the purification process by removing these impurities and enhancing the quality of the final product.
Preparing active ingredients containing an amino group as ammonium chloride salt is important and useful for several reasons. Ammonium salts increase the stability of such compounds, prolonging their shelf life. They also tend to have improved solubility in water compared to their free base counterparts, facilitating formulation and enhancing bioavailability. The ionic interactions of ammonium salts with other charged species can affect the pharmacokinetics and pharmacodynamics of the active ingredient.
Furthermore, the solid-state properties of ammonium salts make them favorable for manufacturing dosage forms like tablets or capsules, due to improved crystallinity, flowability, and compressibility. These factors collectively contribute to the efficacy, stability, and ease of formulation of active ingredients containing an amino group as ammonium chloride salt.
To know more about the Cyalume synthesis refer here :
https://brainly.com/question/32092304#
#SPJ11
how much co(g) is required to completely react with 22.55 g fe2o3? if 15.32 g fe(s) are produced, what is the % yield?
Answers
11.87 g of CO(g) is required to completely react with 22.55 g Fe₂O₃(s) to produce 15.32 g Fe(s). The percent yield of the reaction is 97.15%.
The balanced equation for the reaction between CO(g) and Fe₂O₃(s) is:
Fe₂O₃(s) + 3 CO(g) -> 2 Fe(s) + 3 CO₂( g)
To determine how much CO is required to completely react with 22.55 g of Fe2O3, we need to use the stoichiometry of the reaction.
From the balanced equation, we know that for every 1 mole of Fe₂O₃(s) that reacts, 3 moles of CO(g) are consumed. To convert mass to moles, we use the molar mass of Fe₂O₃(s), which is 159.69 g/mol.
22.55 g Fe2O3 / 159.69 g/mol = 0.1412 moles of Fe2O3
From the balanced equation we know that for every 1 mole of Fe₂O₃(s), 3 moles of CO(g) is required, so we can calculate the moles of CO(g) needed for the reaction:
0.1412 moles Fe₂O₃(s) (3 moles of CO(g) / 1 mole of Fe₂O₃(s)) = 0.4236 moles of CO(g)
To get the mass of CO(g), we can convert the moles to grams using the molar mass of CO(g), which is 28.01 g/mol.
0.4236 moles of CO(g)(28.01 g/mol) = 11.8660 = 11.87 g of CO(g)
To find the % yield, we divide the actual yield (15.32 g Fe(s)) by the theoretical yield (mass of Fe(s) produced from all the Fe₂O₃(s) consumed) and multiply by 100.
Theoretical yield of Fe(s) = mass of Fe₂O₃(s) consumed(mass of Fe(s) produced from 1 mol Fe₂O₃(s) / mol of Fe₂O₃(s) consumed)
= 22.55 g Fe₂O₃(s) (55.85 g Fe(s) / mol Fe(s)) (2 mol Fe(s) / 1 mol Fe₂O₃(s)) (1 mol Fe₂O₃(s) / 159.69 g Fe₂O₃(s)) = 15.77 g Fe(s)
% yield = (15.32 g Fe(s) / 15.77 g Fe(s)) x 100 = 97.15%
So, 11.87 g of CO(g) is required to completely react with 22.55 g Fe₂O₃(s) to produce 15.32 g Fe(s) and the % yield of the reaction is 97.15%.
Learn more about percent yield here: https://brainly.com/question/27918618
#SPJ4