2. for water having a total alkalinity of 1.00 10-3 mol/l and a ph of 10.34 what is the percentage contribution to alkalinity from co32-?
Answers
The percentage contribution of CO₃⁻² to the total alkalinity is approximately 0.058%.
In water chemistry, the total alkalinity (TA) is defined as the sum of all acid-neutralizing substances present in water. These substances can include bicarbonates, carbonates, and hydroxides.
To calculate the percentage contribution of CO₃⁻² to the total alkalinity, we need to determine the concentration of CO₃⁻² in the water.
First, we need to write the equilibrium reaction for CO₃⁻²:
CO₃⁻² + H₂O ↔ HCO³⁻ + OH⁻
We can use the pH of the water to determine the concentration of OH-:
pH = 14.00 - pOH
pOH = 14.00 - 10.34 = 3.66
[OH-] = 10^-pOH = 4.02 x 10^-4 mol/L
Next, we can use the equilibrium constant expression to calculate the concentration of CO32-:
Kw = [H⁺][OH⁻ = 1.00 x 10^-14
Kb = Kw/Ka2 = 4.69 x 10^-11 (for CO₃⁻²)
Kb = [HCO⁻³][OH⁻]/[CO₃⁻²]
Assuming that the concentration of HCO⁻³ is negligible compared to the concentration of CO₃⁻², we can simplify the equation to:
Kb ≈ [OH⁻][CO₃⁻²]
[CO₃⁻²] = Kb/[OH⁻] = (4.69 x 10^-11)/(4.02 x 10^-4) = 1.17 x 10^-7 mol/L
Now we can calculate the contribution of CO₃⁻² to the total alkalinity:
TA = [CO₃⁻²] + 2[CO₃⁻²] + [OH⁻] - [H⁺]
Since the pH is greater than the pKa₂ of CO₃⁻² (10.33), we can assume that all of the CO₃⁻² is present in the form of [CO₃⁻²]⁻² (the second dissociation step), so the equation becomes:
TA = [HCO⁻³] + [CO₃⁻²]⁻² + [OH⁻] - [H⁺]
TA = [HCO⁻³] + 2[CO₃⁻²] + [OH⁻] - [H⁺] + [CO₃⁻²]⁻² - 2[CO32-2]
TA = [HCO⁻³] + [CO₃⁻²] + [OH⁻] - [H⁺] + [CO32-2]
TA = 2[CO₃⁻²] + [OH-]
TA = 2(1.17 x 10^-7) + 4.02 x 10^-4 = 4.03 x 10^-4 mol/L
The contribution of CO₃⁻² to the total alkalinity is:
[CO₃⁻²]/TA x 100% = (2[CO₃⁻²]/TA) x 100% = (2(1.17 x 10^-7)/4.03 x 10^-4) x 100% ≈ 0.058%
Click the below link, to learn more about the Alkalinity of the solution:
https://brainly.com/question/28755002
#SPJ11
Related Questions
1 a) x = 155 ± 4 y = 265 ± 6 What is Δw if w = x + y ? b) x = 155 ± 8 y = 265 ± 8 What is Δw if w = x - y ? c) x = 155 ± 2 y = 265 ± 3 z = 177 ± 3 What is Δw if w = x + 2y - 3z ? Calculate all answer to 2 decimal places. Answer properly for upvotes.
Answers
The term "uncertainty variable" is not a standard term in physics or mathematics.
a) The uncertainty in w is ±10.
b) The uncertainty variable in w is ±0.
c) The uncertainty in w is ±17.
To find the uncertainty (Δw) in each scenario, we need to consider the uncertainties associated with each variable involved in the equation.
a) In the equation w = x + y, we are given that x has an uncertainty of ±4 and y has an uncertainty of ±6. To find the uncertainty in w, we simply add the uncertainties of x and y together:
Δw = ±4 + ±6 = ±10
Therefore, the uncertainty in w is ±10.
b) In the equation w = x - y, we are given that x has an uncertainty of ±8 and y has an uncertainty of ±8. To find the uncertainty in w, we subtract the uncertainty of y from the uncertainty of x:
Δw = ±8 - ±8 = ±0
Therefore, the uncertainty in w is ±0.
c) In the equation w = x + 2y - 3z, we are given that x has an uncertainty of ±2, y has an uncertainty of ±3, and z has an uncertainty of ±3. To find the uncertainty in w, we combine the uncertainties of x, y, and z:
Δw = ±2 + 2(±3) + 3(±3) = ±2 + ±6 + ±9 = ±17
Therefore, the uncertainty in w is ±17.
In summary:
a) Δw = ±10
b) Δw = ±0
c) Δw = ±17
Remember to calculate the answer to 2 decimal places.
To know more about uncertainty, visit:
https://brainly.com/question/33389550
#SPJ11
PLZ HELP GIVING BRAINLEST TO WHO EVER HELPS AND GETS THE RIGHT ANSWER. Plz and tysm
The pavement on a hot day has transformed light energy from______ to heat energy.
1. Radiation
2. Conduction
3. Convection
4.All of the above
Answers
Answer:
4. all the above
Explanation:
It's all of the above because Thermal energy has all three. An example is like a pot of water on the stove boiling. Radiation is coming off of the eye of the stove. Conduction occurs when heat transfers from hot to cold ( like the handle of the pot). Convection occurs in the boiling of the water by rotating causing the heat to rise.
draw the products formed when the following alkene is treated with o3 followed by zn, h2o. be sure to answer all parts.
Answers
The given alkene can undergo ozonolysis in the presence of ozone (O3) followed by reduction with zinc (Zn) and water (H2O) to yield two products.
The ozonolysis reaction cleaves the double bond in the alkene and generates two carbonyl compounds, which can then be reduced by zinc to form aldehydes or primary alcohols depending on the reaction conditions.
The ozonolysis of the given alkene, 2-methyl-2-pentene, results in the formation of two carbonyl compounds: propanal and 2-methylpropanal. These carbonyl compounds can then undergo reduction with zinc and water to form the corresponding aldehydes or primary alcohols.
The reduction of propanal with zinc and water results in the formation of propan-1-ol, which is a primary alcohol. The reaction involves the addition of hydrogen atoms to the carbonyl group of propanal, followed by the removal of the resulting oxygen atom as water. The reduction of 2-methylpropanal with zinc and water results in the formation of 2-methylpropan-1-ol, which is also a primary alcohol. The reduction mechanism is similar to that of propanal, but with the addition of hydrogen atoms to the carbonyl group of 2-methylpropanal instead.
In summary, the products formed when 2-methyl-2-pentene is treated with ozone followed by zinc and water are propan-1-ol and 2-methylpropan-1-ol. These products are formed by ozonolysis of the alkene to generate carbonyl compounds, followed by reduction of the carbonyl compounds to primary alcohols with zinc and water. This reaction demonstrates the versatility of ozonolysis and reduction reactions in synthesizing aldehydes and primary alcohols from alkenes, which are important building blocks in organic chemistry.
To know more about alkene: https://brainly.com/question/13910028
#SPJ4
au lie
3. What are the two categories of pure substances?
a.
b.
I
C.
d.
e.
f.
g.
h.
i.
j.
k.
1.
b.
m.
Answers
pretty sure its d and g
just my opinion
What causes ionic bonding between two atoms?
Answers
Answer:
Ionic bonding happens when an atom of an element gives one or more of its electrons to the other element's atom..it usually takes place between metal and non metal atoms...like in NaCl, Na gives its valence electron to chlorine and completes its own octet. Chlorine accepts the electron and completes its own octet too...but now both the atoms have an opposing charge and hence they attract each other to form an IONIC bond.
Ionic bonds are the strongest of the bonds...here complete transfer of electrons takes place unlike covalent bonds.
HOPE IT HELPED..
:)
Answer:
The electrostatic interaction between an anion and cation
2. At about what temperature will 37 g of both copper(II) sulfate and potassium chloride disse
in 100 g of water?
Answers
The temperature at which 37 g of both copper(II) sulfate and potassium chloride dissolve in 100 g of water is approximately 38 ⁰C.
What is solubility?The solubility of a substance is its ability to be dissolved in water.
Solubility of 37 g of Copper (II) sulfateThe solubility of 37 g of Copper (II) sulfate is determined from the solubility curve of Copper (II) sulfate.
Solubility of 37 g of potassium chlorideThe solubility of 37 g of potassium chloride is determined from the solubility of potassium chloride.
Thus, from the solubility charts, the temperature at which 37 g of both copper(II) sulfate and potassium chloride dissolve in 100 g of water is approximately 38 ⁰C.
Learn more about solubility here: https://brainly.com/question/23946616
#SPJ1


How many atoms are present in 97.34g of zinc?
Answers
To discover the number of atoms in a piece, divide its importance in grams by the periodic table's amu atomic mass, then multiply the result by Avogadro's number: 6.02 x 1023.
What is atoms?An atom is a matter particle that defines a chemical element uniquely. An atom is made up of a central nucleus and one or more negatively charged electrons.The nucleus is positively charged and contains one or more heavy particles called protons and neutrons. An atom exists as an essential particle of value that contains at least one proton.Here are a few atom examples: neon (N) and hydrogen (H) (Ne). Because atoms were once thought to be the smallest things in the universe and could not be divided, the term "atom" comes from the Greek word for indivisible.
To learn more about atoms, refer to:
https://brainly.com/question/23390564
#SPJ4
2Al + 6HCl → 2AlCl3 + 3H2
If the chemical reaction produces 129 grams of AlCl3, how many grams of H2 are also produced?
Answers
Answer: The reaction produces 2.93 g H₂.
M_r: 133.34 2.016
2Al + 6HCl → 2AlCl₃ + 3H₂
Moles of AlCl₃ = 129 g AlCl₃ × (1 mol AlCl₃/133.34 g AlCl₃) = 0.9675 mol AlCl₃
Moles of H₂ = 0.9675 mol AlCl₃ × (3 mol H₂/2 mol AlCl₃) = 1.451 mol H₂
Mass of H₂ = 1.451 mol H₂ × (2.016 g H₂/1 mol H₂) = 2.93 g H₂
Explanation:
you just got home from a run on a hot Atlanta afternoon. you grab a 1.00-liter bottle of water and drink three-quarters of it in one swig. How many moles of water did you consume?
Answers
Answer:
41.67 mol
Explanation:
1 Litre of water = 1000g
Mole = mass / molar mass
Mass of 1 L of water = 1000 g
Molar mass of water (H2O) :
(H = 1, O = 16)
H2O = (1 * 2) + 16 = (2 + 16) = 18g/mol
Amount of water consumed = (3/4) of 1 litre
= (3/4) * 1000g
= 750g
Therefore mass of water consumed = 750g
Mole = 750g / 18g/mol
Mole of water consumed = 41.6666
= 41.67 mol
If block 1 were broken in two equal pieces, what would be the density of each piece? Why?
Answers
Explanation:
The density of the new blocks will be the density of the original block.
For example if the density of the block is 2g/cm³, when the block is broken down into two, each of the new block will have a density of 2g/cm³.
Density is an intensive property of matter. It does not depend on the mass of the substance available. Every sample of a substance have the same value of density.1 point
Given the unbalanced equation below. When the equation is correctly
balanced what is the coefficient in front of O2?*
Al(s) + O2(g)
O2(g) →_Al2O3(s)
1)6
2. 2
3. 3
4. 4
Answers
Answer:
bbecause it make the answer
How many grams of chlorine gas are found in a 12.7 L sample at STP.
Answers
Considering the definition of STP conditions, a mass of 20.10 grams of chlorine gas is found in a 12.7 L sample at STP.
STP conditionsSTP conditions refer to standard temperature and pressure, using 1 atmosphere and 0 °C as reference values for gases. Under these conditions, 1 mole of any gas occupies an approximate volume of 22.4 liters.
Molar massThe molar mass of substance is the amount of mass that a substance contains in one mole.
Mass of chlorine gasIn this case, you can apply the following rule of three: if by definition of STP conditions 22.4 liters of chlorine is occupied by 1 mole of chlorine, 12.7 liters is occupied by how many moles of chlorine?
moles= (12.7 liters× 1 mole)÷ 22.4 liters
moles= 0.567 moles
The molar mass of chlorine is 35.45 g/mole.
Now, you can apply the following rule of three: If by definition of molar mass 1 mole of chlorine has a mass of 35.45 grams, 0.567 moles of chlorine has how much mass?
mass= (0.567 moles× 35.45 grams)÷ 1 mole
mass= 20.10 grams
Finally, a mass of 20.10 grams of chlorine gas is present.
Learn more about STP and molar mass:
brainly.com/question/3773297
brainly.com/question/9901446
brainly.com/question/12695086
#SPJ1
What is the molar mass of carbon?
Answers
The_____gene shows up even when inherited only from one parent.
Answers
Answer:
The Alleles gene shows up even when inherited only from one parent
Explanation:
The two alleles in a gene pair are inherited, one from each parent. Alleles interact with each other in different ways. These are called inheritance patterns.
i hope this helped
You are going to carry out a chemical reaction in which you need 16 g of oxygen for every 7.0 g of
nitrogen that will be used. If you have 0.554 kg of oxygen, how many milligrams of nitrogen do you
need?
Answers
Answer: To solve this problem, we can use the given information to set up a proportion. The ratio of oxygen to nitrogen in the reaction is 16 g : 7.0 g, or 8 : 3.5. We can set up a proportion using these ratios as follows:
(3.5) x = (8) 0.554 kg
We can solve this proportion by cross multiplying to find the value of x, which is the amount of nitrogen needed:
3.5 * 0.554 kg = 8 * x
1.947 kg = 8 * x
x = 0.24125 kg
We can convert this value to milligrams by multiplying it by 1,000,000:
x = 0.24125 kg * 1,000,000 mg/kg
= 241,250 mg
Therefore, the amount of nitrogen needed for the chemical reaction is approximately 241,250 milligrams.
Calculate the Molar Mass of Radon pentaiodide (Rnls)
857
349
1237
Answers
Explanation:
222.01758 g/mol
3.1Computed Properties
Which element would be the most suitable to make a spoon that will melt in
your hot drinks?
A) aluminium
melting point: 660°C
boiling point: 2470°C
B) argon
melting point: -189°C
boiling point:-186°C
C) bromine
melting point: -7°C
boiling point: 59°C
D) gallium
melting point: 30°C
boiling point: 2400°C
E) lithium
melting point: 180°C
boiling point: 1330°C
F)mercury
melting point: -39°C
boiling point:357°C
Answers
Answer:
it's A) or E)
others are not suitable cuz their melting point is weak.
Answer:
Gallium
Explanation:
Because the melting point is low and the drinks are usually around 60-70 degrees Celsius so it wil melt
An ideal gas has a volume of 18.25 L at a temperature of 15.9 °C. The temperature of the gas is raised to 40.7 °C while the number of moles and the pressure of the gas are kept constant. What is the new volume of the gas (in L)?
B: An ideal gas has a volume of 10.8 L at a temperature of 25.0 °C and a pressure of 60 atm. The pressure of the gas is reduced to 370.0 mmHg, but the temperature and number of moles of the gas are kept constant. What is the new volume of the gas (in L)?
Answers
Answer:
V₂ = V₁ / T₁ * T₂ . If you prefer to set the final volume and want to estimate the resulting temperature, then the equation of Charles' law changes to: T2=T1/T1 multiplied by v^2.
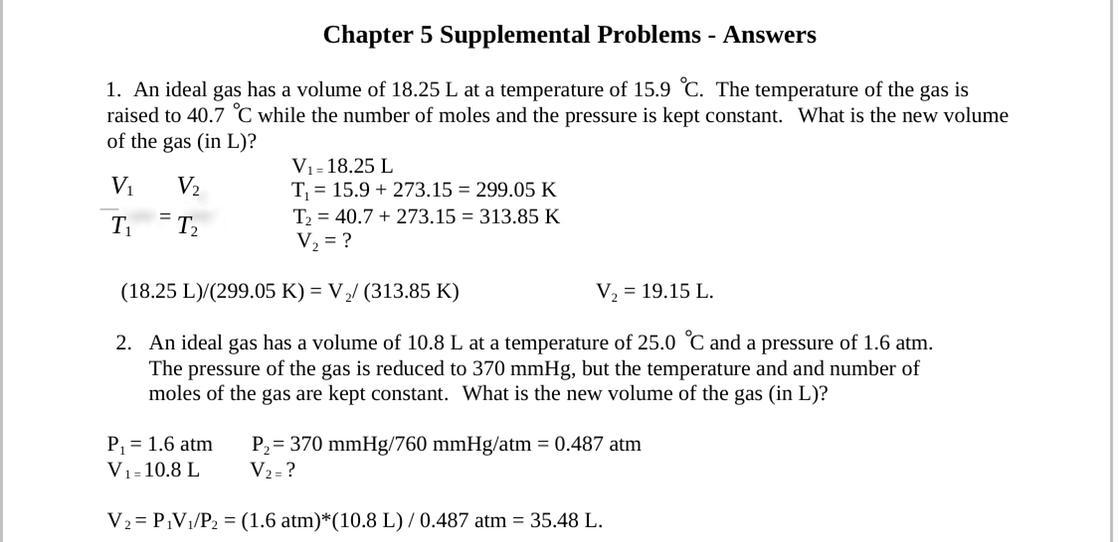
I need help, plz and thank you.

Answers
Which of the following atoms will have the same electron configuration?
I. N−3
II. O+2
III. F−1
IV. Li−1
V. Be+2
Answers
Answer:
I. N−3
III. F−1
Explanation:
A positive charge indicates a loss of electrons whereas a negative charge denotes electron gain.
For two species to have the same electron configuration, they must have the same number of electrons within their atomic space.
So, the solution to this problem will be two species with the same number of electrons:
Nitrogen in neutral state has 7 electrons, on gaining 3 the electrons becomes 10
Fluorine in neutral state has 9 electrons, on gaining 1 more, it will have 10 electrons.
Choose the terms that correctly complete the paragraph.
Mr. Tracy drove to the nursery to buy plants for his garden. His trip involved several examples of chemical energy. The chemical energy contained in {Blank} changed into energy to run the car. The plants store chemical energy produced during {Blank} . Mr. Tracy had the energy to pick up the plants and carry them to the car because of the chemical energy stored in {Blank} .
Answers
The chemical energy contained in gas changed into energy to run the car. The plants store chemical energy produced during photosynthesis. Mr. Tracy had the energy to pick up the plants and carry them to the car because of the chemical energy stored in foods.
Energy conversion in systemsMr. Tracy drove to the nursery to buy plants for his garden. While driving, the chemical energy stored in the gas present in the gas tank in his car is burned to produce energy that drives the engine of the car.
The plants that Mr. Tracy was going to buy store chemical energy through the production of carbohydrates via the process of photosynthesis. Mr. Tracy had the energy to pick up the plants and carry them to the car because of the energy stored in foods.
The foods are oxidized during respiration to unlock the energy and convert them to usable energy by the body, usally in the form of ATP. Respiration involves series of steps, including the conversion of glucose to pyruvate molecules and the conversion of the pyruvates to carbon dioxide and water.
More on energy conversion can be found here: https://brainly.com/question/12503064
#SPJ1
is there evidence of the diffusion of iodine molecules? if so, what is the evidence and in which direction did iodine molecules diffuse?
Answers
Yes, Water moves through osmosis as it transitions from a high concentration to a low concentration. Osmosis via the dialysis tube was demonstrated by the fact that there was an increase in liquid in the dialysis bag and a decrease in water and iodine solution in the cup.
Osmosis is the passage of water through a membrane, and movement occurs continuously. A membrane is not required for diffusion, which is the movement of molecules.
They both transition from a high concentration to a low concentration, they don't require energy, and they happen in both plants and animals.
Learn more about diffusion here:
https://brainly.com/question/94094
#SPJ4
Which is the primary energy-carrying molecule in metabolic pathways?
A) AMP B) ATP C) NADH D) Acetyl CoA E) FADH2
Answers
ATP (adenosine triphosphate) is the primary energy-carrying molecule in metabolic pathways.
ATP, or adenosine triphosphate, is the primary energy-carrying molecule in metabolic pathways. It is often referred to as the "energy currency" of the cell because it stores and releases energy for cellular processes. ATP consists of a nucleotide base (adenine), a sugar molecule (ribose), and three phosphate groups. The high-energy phosphate bonds between the phosphate groups make ATP an excellent source of readily available energy.
In Metabolic pathways, ATP plays a crucial role in energy transfer. When ATP is hydrolyzed, meaning one of its phosphate groups is broken off, it releases energy. This energy is used to drive various cellular processes, such as active transport, DNA synthesis, and muscle contraction. ATP is continuously regenerated through cellular respiration, where energy-rich molecules like glucose are broken down to produce ATP.
Overall, ATP serves as the primary energy carrier in metabolic pathways, providing the necessary energy for cellular activities through its phosphate bonds.
To learn more about ATP refer:
https://brainly.com/question/30770497
#SPJ11
Which postulate of daltons atomic model was later changed and why
Answers
a student conducts an experiment to determine of adding salt on the boiling temperature of water what is the dependent variable
Answers
Answer:
Take a screenshot
Explanation:
Help which one will it be

Answers
Answer:
D
Explanation:
Answer:
the answer is D I did that one in 5th grade
In a reaction, 2mol of oxygen reacts with 8g of ethane to produce carbon dioxide and water, calculate the mass of carbon dioxide formed in this reaction. 2C2H6 +7O2 > 4CO2 +6H2O
Answers
Answer:
The mass of carbon dioxide formed in the reaction is 23.47 grams.
Explanation:
The balanced reaction is:
2 C₂H₆ +7 O₂ ⇒ 4 CO₂ + 6 H₂O
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of each compound participate in the reaction:
C₂H₆: 2 moles O₂: 7 moles CO₂: 4 molesH₂O: 6 molesBeing the molar mass of the compounds:
C₂H₆: 30 g/moleO₂: 16 g/mole CO₂: 44 g/moleH₂O: 18 g/moleBy reaction stoichiometry, the following mass amounts of each compound participate in the reaction:
C₂H₆: 2 moles* 30 g/mole= 60 gO₂: 7 moles* 16 g/mole= 112 gCO₂: 4 moles* 44 g/mole= 176 gH₂O: 6 moles* 18 g/mole= 108 gThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
To determine the limiting reagent, you can use a simple rule of three as follows: if by stoichiometry of the reaction 7 moles of O₂ react with 60 g of C₂H₆, 2 moles of O₂ will react with how much mass of C₂H₆?
\(mass of C_{2}H_{6} =\frac{2 moles of O_{2}* 60 grams of C_{2}H_{6} }{7 moles of O_{2}}\)
mass of C₂H₆= 17.14 grams
But 17.14 grams of C₂H₆ are not available, 8 grams are available. Since you have less moles than you need to react with 2 moles of O₂, ethane C₂H₆ will be the limiting reagent.
Then, it is possible to determine the amount of mass of CO₂ produced by another rule of three: if by stoichiometry 60 grams of C₂H₆ produce 176 grams of CO₂, how many mass of CO₂ will be formed if 8 grams of C₂H₆ react?
\(mass of CO_{2} =\frac{8 grams of C_{2}H_{6}* 176 grams of CO_{2} }{60 grams of C_{2}H_{6}}\)
mass of CO₂= 23.47 grams
The mass of carbon dioxide formed in the reaction is 23.47 grams.
for the pair of molecules below state the strongest intermolecular force that can form between them (ion-dipole; dipole-dipole; dipole-induced dipole; hydrogen bond; van der waals) nacl and ch3oh
Answers
The strongest intermolecular force that can form between NaCl (sodium chloride) and CH₃OH (methanol) is ion-dipole interaction.
NaCl is an ionic compound composed of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). On the other hand, CH₃OH is a polar molecule with a partial positive charge on the carbon atom and partial negative charges on the oxygen and hydrogen atoms.
Ion-dipole interactions occur between ions and polar molecules. In this case, the positive charge on the sodium ion can attract the partial negative charge on the oxygen atom of methanol, while the negative charge on the chloride ion can attract the partial positive charge on the carbon atom or hydrogen atoms of methanol.
Dipole-dipole interactions and hydrogen bonding, which are also intermolecular forces, are typically weaker than ion-dipole interactions. Dipole-induced dipole and van der Waals forces are even weaker forces.
Therefore, the strongest intermolecular force that can form between NaCl and CH₃OH is ion-dipole interaction.
You can learn more about intermolecular force at: brainly.com/question/31797315
#SPJ11
A man earned a $7,500 commission by selling a coin-operated laundry business, which has six more years before the expiration of its present lease. The man
Answers
A man earned a $7,500 commission by selling a coin-operated laundry business, which has six more years before the expiration of its present lease. To evaluate whether or not this sale was worth it, we must calculate the total amount of money that the laundry business will generate in the remaining six years of its current lease.
This can be calculated as follows: Monthly revenue from laundry business = $2,500Commission paid to man = $7,500Revenue generated in one year = Monthly revenue x 12 = $2,500 x 12 = $30,000Revenue generated in six years = Revenue generated in one year x 6 = $30,000 x 6 = $180,000Therefore, the laundry business will generate $180,000 in the remaining six years of its lease. Given that the man earned a $7,500 commission by selling the business, this sale was definitely worth it as the amount of money generated from the business in the remaining six years of its lease far exceeds the commission paid to the man.
The man earned a $7,500 commission by selling a coin-operated laundry business that still had six years remaining on its current lease. To determine whether this sale was worthwhile, we must first calculate the amount of money the laundry business will generate during the remaining six years of its current lease. We can do this by determining the laundry business's monthly revenue and multiplying it by 12 to get the yearly revenue.
After that, we can multiply the yearly revenue by 6 to obtain the total revenue for the next six years.Since the monthly revenue from the laundry business is $2,500, the yearly revenue is $30,000 (2,500 x 12 = 30,000). The business will earn $180,000 in the six years remaining on its lease (30,000 x 6 = 180,000).Given that the man received $7,500 in commission by selling the business, we can determine that this was a profitable sale since the total revenue generated from the business in the next six years far exceeded the commission paid to the man.
It is clear that selling the coin-operated laundry business was worth it since the total revenue generated in the remaining six years of the lease was $180,000, whereas the man earned only $7,500 in commission for selling the business. This demonstrates that this was a profitable sale and an excellent business decision.
To know more about revenue :
brainly.com/question/27325673
#SPJ11
The man in question is a commission-based sales agent who earned $7,500 by selling a coin-operated laundry business. The laundry business is still leased for another six years before the lease expires.This information alone is insufficient to make any meaningful conclusions about the man in question.
However, we can surmise a few things based on the information provided.First, we know that the man is a sales agent, and he likely works for a brokerage or agency that specializes in selling businesses. We can also assume that he is experienced in selling businesses, and that he has a good reputation in the industry, since he was able to sell the coin-operated laundry business and earn a commission of $7,500. Finally, we can speculate that he has good communication and negotiation skills, since he was able to successfully negotiate the sale of the business and earn a commission.The information provided does not give us any other details about the man in question, such as his age, background, or personal interests. However, based on the information we do have, we can assume that he is a successful sales agent who is well-regarded in his industry.For such more question on communication
https://brainly.com/question/28153246
#SPJ8
Which of these weak bases is the weakest electrolyte in aqueous solution? ethyl amine, Kb = 4.3 x 10-4 O aniline, Kp = 4.0 x 10-10 O hydrazine, Kp = 8.5 x 10-7 O trimethyl amine, Kb = 6.5 x 10-5
Answers
Among the given weak bases, aniline is the weakest electrolyte in an aqueous solution.What is an electrolyte?An electrolyte is a substance that conducts electricity in an aqueous solution or in a molten state.
In water, they break up into ions and conduct electricity. Electrolytes may be categorized into two types: strong and weak electrolytes. Strong electrolytes dissociate completely into ions in aqueous solution, whereas weak electrolytes only partially dissociate into ions and exist in equilibrium with undissociated molecules. In the given weak bases, aniline is the weakest electrolyte.
Here's how to solve the problem: Aniline has a Kp of 4.0 × 10-10, which is the smallest value of Kp among all the given weak bases. Therefore, aniline is the weakest electrolyte in an aqueous solution.
Read more about electrolyte here;https://brainly.com/question/17089766
#SPJ11