Answers
The empirical formula for \(C_3_6 H_9\) is \(C_4H\).
Chemical formulas that give only the relative number of atoms of each type in a molecule are called empirical formulas. The subscripts in an empirical formula are always the smallest possible whole-number ratios.
Look at the ratio of these two atoms in the compound
C : H
36: 9
Divide the ratio by 9 to get simple ratio:-
C : H
36/9 : 9/9
4 : 1
For \(C_3_6 H_9\) , the smallest whole number ratio is 4:1. So, the empirical formula for \(C_3_6 H_9\) is \(C_4H\).
So, the empirical formula for \(C_3_6 H_9\) is \(C_4H\).
To know more about:-
https://brainly.com/question/14044066

Related Questions
4- The standard potential of cell: Sn/Sn²+||Cr³+/Cr is −0.60V.what is the standard
reduction potential of the Cr³+/Crelectrode? Es = -0.14V
Sn²+
(b) +0.74V
(c) -0.88V
(d) -0.74V
(a) +0.88V
Answers
The standard reduction potential of the Cr³+/Cr electrode is -0.46V. None of the option is correct.
To determine the standard reduction potential of the Cr³+/Cr electrode, we can use the Nernst equation, which relates the standard reduction potential to the cell potential under non-standard conditions. The Nernst equation is given by:
E = E° - (0.0592/n) * log(Q)
where E is the cell potential, E° is the standard reduction potential, n is the number of electrons transferred in the half-reaction, and Q is the reaction quotient.
In this case, we have the standard potential of the cell as −0.60V. We know that the standard reduction potential of the Sn/Sn²+ electrode is -0.14V. Therefore, the reduction potential of the Cr³+/Cr electrode can be calculated as:
E = -0.60V - (-0.14V)
E = -0.60V + 0.14V
E = -0.46V
Therefore, the standard reduction potential of the Cr³+/Cr electrode is -0.46V.
For more such question on standard reduction potential visit;
https://brainly.com/question/31482299
#SPJ8
Robert Delaunay's Homage to Blériot (1914) was inspired by
O the invention of stroboscopic photography
the construction of the Eiffel Tower
his wife's new dress designs
the first flight across the English channel
Answers
Answer:
Robert Delaunay's Homage to Blériot (1914) was inspired by the first flight across the English channel.
Which of these does not accurately describe the Wright brothers' first
airplane?
A. It had wings that flapped,similar to birds wings
B.it had powerful yet exceptionally lightweight engines
C.it was created after various designs were tested in a wind tunnel
D.it was created using the engineering process
Answers
Answer: A
Explanation:
it had wings that flapped, similar to birds' wings.
KCIO3 -> KCI + 02
How many moles of KCI are produced if 6743 grams of KCIO3 decomposes?
Answers
55.03 moles of KCI are produced when 6743 grams of \(KClO_{3}\) decomposes
To determine the number of moles of KCl produced when 6743 grams of \(KClO_{3}\) decomposes, we need to use the concept of molar mass and the balanced chemical equation.
First, let's calculate the molar mass of \(KClO_{3}\)
The molar mass of potassium (K) is approximately 39.10 g/mol.
The molar mass of chlorine (Cl) is approximately 35.45 g/mol.
The molar mass of oxygen (O) is approximately 16.00 g/mol.
So, the molar mass of \(KClO_{3}\) is:
(39.10 g/mol) + (35.45 g/mol) + (3 * 16.00 g/mol) = 122.55 g/mol.
Now, we need to calculate the number of moles of \(KClO_{3}\):
Number of moles = Mass / Molar mass
Number of moles = 6743 g / 122.55 g/mol = 55.03 mol.
According to the balanced chemical equation:
2\(KClO_{3}\) -> 2 KCl + 3 O2,
we can see that for every 2 moles of \(KClO_{3}\), we obtain 2 moles of KCl.
Therefore, the number of moles of KCl produced will be equal to the number of moles of \(KClO_{3}\) since the ratio is 1:1. Thus, 55.03 moles of KCl will be produced.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ11
if you are using a formula where you need the change in temperature, explain why it is not important whether your temperatures are both in Kelvin or Celsius?
Answers
Answer:
This is because, Kelvins and Celcius degrees both agree at fixed points i.e; the lower fixed point and upper
5 ways light changes objects

Answers
2) it could make the object hot if the light stays on it for long enough
3) it forms energy, such as light helps makes plants grow
4) it can make it change colors, such as if you put a flashlight underneath a balloon making it a lighter colour
5) it can reflect of the objects, such as a mirror
Which two the following functional groups does the amino acid have according to the picture? ( worth 50 points <3)

Answers
The two functional groups that the aminoacid has according to the picture are amine and carboxyl.
What is a functional group?In chemistry and related areas, a functional group can be defined as a group of atoms bonded in a specific molecule that can affect the was the molecule reacts or the specific behavior of it.
In the case of the molecule presented, which is an amino acid, two functional groups can be identified:
An amine group: This includes the N atom bonded to the two hydrogens.A carboxyl group: This includes the terminal carbon linked to two oxygen atoms and a hydrogen atom.Learn more about functional groups in https://brainly.com/question/1356508
#SPJ1
If 1.2 g of ammonium bicarbonate is treated with .75 grams of sodium chloride, 63.0075 grams sodium bicarbonate will be produced. In yhis reaction NacI is limiting reagent true or false
Answers
NaCl as a limiting reactant ⇒ true
Further explanationGiven
1.2 g NH₄HCO₃
0.75 g NaCl
63.0075 g NaHCO₃
Required
The limiting reactant
Solution
Reaction
NH₄HCO₃ + NaCl ⇒NH₄Cl + NaHCO₃
mol NH₄HCO₃ :
= 1.2 g : 79,056 g/mol
= 0.015
mol NaCl :
= 0.75 : 58.5
= 0.013
NaCl as a limiting reactant(smaller mol)
Indicate the subatomic particle described by each of the statements. A statement may describe more than one particle. possesses a negative charge has no charge has a mass slightly less than that of a neutron has a charge equal to but opposite in sign to that of an electron is not found in the nucleus Answer Bank has a positive charge proton neutron can be called a nucleon electron is the heaviest of the three subatomic particles has a relative mass of 1836 if the mass of an electron is 1 has a relative mass of 1839 if the mass of an electron is 1
Answers
a)possesses a negative charge - electron
b)has no charge - neutron
c)has a mass slightly less than that of a neutron - proton
d)has a charge equal to, but opposite in sign from, that of an electron - proton.
What has a negative charge?
Electrons have a negative charge. The charge on the proton and electron are exactly the same size but opposite. Neutrons have no charge. Since opposite charges attract, protons and electrons attract each other.
What are the four properties of neutrons?
Neutrons are neutral particles – with no net electric charge.
Neutrons have a non-zero magnetic moment.
Free neutrons (outside a nucleus) are unstable and decay via beta decay.
Is a neutron's mass slightly less than a proton's?
The mass of a neutron is slightly greater than the mass of a proton, which is 1 atomic mass unit (AMU).
Is the charge of an electron opposite to the charge of a proton?
Electrons and protons have equal and opposite charges. The magnitude of this charge is 1.6×10−19 Coulomb.
To know more about protons, neutrons, and electrons:
https://brainly.com/question/29248303
#SPJ4
Guysss how to explain nuclear chemistry? And define nuclear chemistry ?
Answers
Answer:
How do amoeba respire.
Define Diffusion.
What is the mass of CO2 lost at 20 min from the limestone sample?
How much CO2, in moles, was lost at 20 min from the limestone sample?
How much CO3 2- was lost at 20 min from the limestone sample?
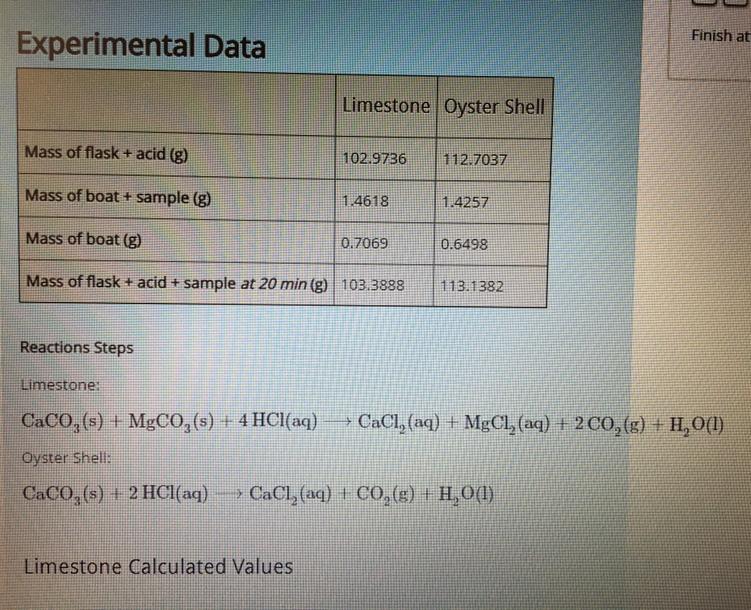
Answers
Answer:
The mass, CO2 and CO3 from the limestone sample is discussed below in details.
Explanation:
(A) mass loss of sample of limestone after 20 min
= 0.8437g-0.5979g = 0.2458 g
From the given reaction of limestone, 2 mol of the sample gives 2 moles of CO 2.
Therefore
184.4 g ( molar mass of limestone) gives2× 44 g of carbon dioxide.
1 g of sample gives 88/184.4 g of carbon dioxide
Hence 0.2458 g sample gives
= 88/184.4 × 0.2458 g = 0.117 g carbon dioxide
(B) mole of CO 2 lost = weight/ molar mass
= 0.117 g / 44 g/mol =0.0027 mole
(C). 1 mol of limestone contain 2 mol of carbonate ion
From the reaction we know that carbonate ion of limestone is converted into carbondioxide
Hence lost carbonate ion = 0.2458 g
(D) we know that
1 mol limestone contain 1mol CaCO 3
Hence in sample present CaCO 3
= 1mole / 184.4 g × 0.8437 g= 0.00458 mol CaCO3
1 answer
.....................................

Answers
Answer:
Signal noise will reduce the information in the transmitted signal.
Explanation:
Signal noise is the most likely factor that will reduce the information in a transmitted signal because:
Signal noise is any unwanted interference that degrades a communication signal.Signal noise can interfere with both analog and digital signals, and it can cause distortion of the signal, which can lead to errors in the information that is being transmitted.Two factors that determine kinetic energy are
speed and distance
mass and speed
mass and distance
distance and time
Answers
PLS ANSWER BOTH QUESTIONS I ONLY HAVE AN HOUR PLSSSS

Answers
The number of moles of carbondioxide that will be produced in the combustion of methane is 3 moles (option D).
How to calculate moles using stoichiometry?Stoichiometry refers to the study and calculation of quantitative (measurable) relationships of the reactants and products in chemical reactions (chemical equations).
According to this question, methane undergoes combustion in air to produce carbondioxide and water. Methane is the limiting reactant because the other reactant is oxygen of the air which is always present in excess.
Based on the chemical equation given above, 1 mole of methane produces 1 mole of carbondioxide.
This means that 3 moles of methane will produce 3 moles of carbondioxide.
Learn more about stoichiometry at: https://brainly.com/question/9743981
#SPJ1
A 13.0 ml sample of an acid requires 37,3 ml of 0.303N NaOH for neutralization. Calculate the normality of the acid.
Answers
The amount of 0.303N NaOH needed to neutralize a 13.0 ml sample of acid is 37,3 ml. Acid is 0.823N normal, according to the standard.
Explain about the neutralization.The idea of neutralization, according to which an acid reacts with a base to create salt and water. By comparing the molarity of the base to the amount of base needed for neutralization, it is possible to calculate the molarity of the acid. Once the molarity and the acid's valence (or charge) have been multiplied, the acid's normalcy can be determined.
By deducting the volume of NaOH (37.3 ml) from the volume of the acid sample in Step 1, you can calculate the amount of acid that was utilized in the neutralization procedure (13.0 ml).
Consequently, 13.0 ml to 37.3 ml
= -24.3 ml
Use the equation moles = normality x volume to determine the number of moles of acid that were used in the process.
Consequently, moles equal 0.303N x -24.3 ml.
= -7.33 moles
Determine the acid's normality by multiplying the volume by the formula normalcy = moles.
As a result: normalcy = -7.33 moles / 13.0 ml
= -0.823N
To learn more about neutralization, visit
brainly.com/question/15347368
#SPJ1
7) How many molecules of CO2 are in 2.5 L at STP?
Answers
By using the ideal gas law and Avogadro's number, we find that there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
To determine the number of molecules of CO2 in 2.5 L at STP (Standard Temperature and Pressure), we can use the ideal gas law and Avogadro's number.
Avogadro's number (N_A) is a fundamental constant representing the number of particles (atoms, molecules, ions) in one mole of substance. Its value is approximately 6.022 × 10^23 particles/mol.
STP conditions are defined as a temperature of 273.15 K (0 °C) and a pressure of 1 atmosphere (1 atm).
First, we need to convert the volume from liters to moles of CO2. To do this, we use the ideal gas law equation:
PV = nRT,
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we have STP conditions, we can substitute the values:
(1 atm) × (2.5 L) = n × (0.0821 L·atm/(mol·K)) × (273.15 K).
Simplifying the equation:
2.5 = n × 22.4149.
Solving for n (the number of moles):
n = 2.5 / 22.4149 ≈ 0.1116 moles.
Next, we can calculate the number of molecules using Avogadro's number:
Number of molecules = n × N_A.
Number of molecules = 0.1116 moles × (6.022 × 10^23 particles/mol).
Number of molecules ≈ 6.72 × 10^22 molecules.
Therefore, there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
For more such questions on ideal gas law visit:
https://brainly.com/question/27870704
#SPJ8
4 Fe(s) + 3 02(g)
--> 2 Fe₂O3(s)
1. What is the oxidation state of iron (Fe) in the reactant Fe(s)?
2. What is the oxidation state of oxygen (O) in the reactant O2(g)?
3. What is the oxidation state of iron (Fe) in the product Fe2O3(s)?
4. What is the oxidation state of oxygen (O) in the product Fe2O3(s)?
5. In this reaction, iron is... (oxidized or reduced?)
6. In this reaction, oxygen is... (oxidized or reduced?)
7. What was the oxidizing agent in this reaction: Fe(s) or O2(g)?
Answers
The oxidation number of reactant Fe is 0 while the oxidation number of iron in the product is +3
What s a redox reaction?The term redox reaction implies a reaction in which there is an increase in the oxidation number of a specie and the decrease in the oxidation number of another specie.
Now we have the answers as follows;
1) The oxidation number of reactant Fe is 0
2) The oxidation number of reactant oxygen is 0
3) The oxidation number of iron in the product is +3
4) The oxidation number of oxygen in the product is -2
5). Iron is oxidized in the reaction
6) Oxygen is reduced in the reaction
7) The oxidizing agent in this case is the oxygen atom
Learn ore about redox reaction:https://brainly.com/question/13293425
#SPJ1
CHEM FINAL TOMORROW, NEED IMMEDIATE HELP!!! There are a few topics I don't understand, if someone could give a short explanation of how to do these kinds of problems, it would help so much. I'll be posting a few more of these on my page, so feel free to check those out if you would like. Thanks!

Answers
The mass of \(O_2\) needed to produce 8.65 x \(10^{23\) atoms of silver is 22.96 grams.
Stoichiometric calculationIn the given balanced equation, it is stated that 2 moles of \(Ag_2O\) produce 4 moles of Ag and 1 mole of \(O_2\).
First, let's calculate the number of moles of Ag:
8.65 x \(10^{23\) atoms of Ag / (6.022 x \(10^{23\) atoms/mol) = 1.435 moles of Ag
Since 2 moles of \(Ag_2O\) produce 1 mole of \(O_2\), the number of moles of \(O_2\) needed is half of the moles of \(Ag_2O\).
Number of moles of \(O_2\)= 1.435 moles of \(Ag_2O\) / 2 = 0.7175 moles
Now, we'll use the molar mass of \(O_2\) to find the mass:
Molar mass = 32.00 g/mol
Mass of \(O_2\) = number of moles × molar mass
= 0.7175 moles × 32.00 g/mol = 22.96 g
Therefore, the mass of \(O_2\) needed to produce 8.65 x \(10^{23\) atoms of silver is 22.96 grams.
More on stoichiometric calculations can be found here: https://brainly.com/question/27287858
#SPJ1
Question 1
Given the equation: Q = mcAT
Q = heat (in Joules)
m = mass (in grams)
C = 4.18 (specific heat capacity)
AT change in temperature (°C)
How many Joules of heat energy are absorbed when 200 grams of water are heated from 20 C to 60 C.

Answers
The amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
To find the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C, we can use the equation Q = mcAT.
First, we need to find the value of m, which is the mass of the water in grams. In this case, it is given as 200 grams.
Next, we need to find the value of AT, which is the change in temperature in degrees Celsius.
This can be calculated by subtracting the initial temperature from the final temperature, which gives us 60 C - 20 C = 40 C.
The specific heat capacity of water, C, is given as 4.18 Joules per gram per degree Celsius.
Now we can plug in the values into the equation:
Q = mcAT
Q = (200 g) x (4.18 J/g°C) x (40°C)
Q = 33,440 J
Therefore, the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
for more such question on heat energy
https://brainly.com/question/25603269
#SPJ8
Calculate the vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane (C₂H4Br₂, Pº=127 torr)
in 1.80 mol of liquid dibromopropane (C3H6Br2, P=173 torr).
torr
Answers
The vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane the vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane is 164.83 torr.
What is vapor pressure ?The term vapor pressure is defined as the tendency of a material to change into the vapour state, and it increases with temperature.
For calculating mole fraction of C₂H₄Br₂ as follows
X C₂H₄Br₂ = moles of C₂H₄Br₂ / moles of C₂H₄Br₂ + moles of C₃H₆Br₂
= 0.3 / 0.3 + 1.80
= 0.14
For calculating mole fraction of C₃H₆Br₂ as follows:
XC₃H₆Br₂ = moles of C₃H₆Br₂ / moles of C₂H₄Br₂ + moles of C₃H₆Br₂
= 1.80 / 2.1
= 0.85
For calculating total vapor pressure as follows:
P total = [ ( 0.14 × 127) + (0.85 × 173) ]
= 17.78 + 147.05
= 164.83 torr
Thus, The vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane the vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane is 164.83 torr.
To learn more about the vapor pressure, follow the link;
https://brainly.com/question/11864750
#SPJ1
Surgical instruments may be sterilized by heating at 170°C for 1.5 hours. Which of the following shows the correct conversion of 170°C to kelvins? *
A. -103 K
B. 73 K
C. 443 K
D. 621 K
Answers
The declaration that shows the correct conversion of 170°C to kelvins is 443 K. Thus, the correct option for this question is C.
How to convert degree Celcius into kelvin?The conversion of Celcius to kelvin required the utilization of the formula which is as follows:
Kelvin = Degree Celcius + 273.15.Sterilization of surgical instruments at 170°C for 1.5 hours helps in the process of elimination of contaminated or unwanted impurities from the surface of the instruments in order to conduct error-free experiments.
According to the question,
The temperature of sterilization is = 170°C.
Now, it is required to convert 170°C into kelvin.
∴ Kelvin = 170°C + 273.15 = 443.15 ≅ 443 K.
Therefore, the declaration that shows the correct conversion of 170°C to kelvins is 443 K. Thus, the correct option for this question is C.
To learn more about Sterilization, refer to the link:
https://brainly.com/question/10432738
#SPJ2
15.0 L of an ideal gas at 298 K and 3.36 bar are heated to 350 K with a new pressure of 4.40 atm. What is the new volume in litres?
Answers
Answer:
13.3 L
Explanation:
Step 1: Given data
Initial pressure (P₁): 3.36 barInitial volume (V₁): 15.0 LInitial temperature (T₁): 298 KFinal pressure (P₂): 4.40 atmFinal volume (V₂): ?Final temperature (T₂): 350 KStep 2: Convert P₁ to atm
We will use the conversion factor 1 atm = 1.01325 bar.
3.36 bar × (1 atm / 1.01325 bar) = 3.32 atm
Step 3: Calculate V₂
We will use the combined gas law.
P₁ × V₁/T₁ = P₂ × V₂/T₂
V₂ = P₁ × V₁ × T₂/T₁ × P₂
V₂ = 3.32 atm × 15.0 L × 350 K/298 K × 4.40 atm
V₂ = 13.3 L
What functional groups are in ch2=chch2oh?
Answers
Explanation:
H
|
H-C=C-C-OH
| | |
H H H
in this organic compound the functional groups are OH and alkene
prop-2-enol
since it has a double bond, alkene is the functional group. and also it has OH group so hydroxyl group also the other functional group
Rhodium has an atomic radius of 0.1345 nm and density of 12.41 gm/cm3 . Determine whether it has an FCC or BCC crystal structure.
Answers
Answer:
FCC.
Explanation:
Hello,
In this case, since the density is defined as:
\(\rho =\frac{n*M}{Vc*N_A}\)
Whereas n accounts for the number of atoms per units cell (2 for BCC and 4 for FCC), M the atomic mass of the element, Vc the volume of the cell and NA the Avogadro's number. Thus, for both BCC and FCC, the volume of the cell is:
\(Vc_{BCC}=(\frac{4r}{\sqrt{3} } )^3=(\frac{4*0.1345x10^{-7}cm}{\sqrt{3} } )^3=2.997x10^{-23}cm^3\\\\Vc_{FCC}=(2\sqrt{2}r)^{3} =(2\sqrt{2} *0.1345x10^{-7}cm)^3=5.506x10^{-23}cm^3\)
Hence, we compute the density for each crystal structure:
\(\rho _{BCC}=\frac{n_{BCC}*M}{Vc_{BCC}*N_A}=\frac{2*102.9g/mol}{2.337x10^{-23}cm^3*6.022x10^{23}/mol} =14.62g/cm^3\\\\\rho _{FCC}=\frac{n_{FCC}*M}{Vc_{FCC}*N_A}=\frac{4*102.9g/mol}{5.506x10^{-23}cm^3*6.022x10^{23}/mol} =12.41g/cm^3\)
Therefore, since the density computed as a FCC crystal structure matches with the actual density, we conclude rhodium has a FCC crystal structure.
Regards.
Space shuttles are made out of three main parts: rocket boosters, a fuel tank, and a(n) ___________.
Answers
Answer:
Orbiter
Explanation:
Space shuttles are made out of three main parts: rocket boosters, a fuel tank, and orbiter (the part that resembles an airplane
Identify reactions types and balancing equations

Answers
Balance the following chemical equations:
1. N2 + 3 H2 → 2 NH3
Ex: Synthesis reaction
2. 2 KClO3 → 2 KCl + 3 O2
Single Replacement reaction
3. 2 NaF + ZnCl2 → ZnF2 + 2 NaCl
Decomposition reaction
4. 2 AlBr3 + 3 Ca(OH)2 → Al2(OH)6 + 6 CaBr2
Double Replacement reaction
5. 2 H2 + O2 → 2 H2O
Combustion reaction
6. 2 AgNO3 + MgCl2 → 2 AgCl + Mg(NO3)2
Synthesis reaction
7. 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Decomposition reaction
8. C3H8 + 5 O2 → 3 CO2 + 4 H2O
Combustion reaction
9. 2 FeCl3 + 6 NaOH → Fe2O3 + 6 NaCl + 3 H2O
Double Replacement reaction
10. 4 P + 5 O2 → 2 P2O5
Synthesis reaction
11. 2 Na + 2 H2O → 2 NaOH + H2
Single Replacement reaction
12. 2 Ag2O → 4 Ag + O2
Decomposition reaction
13. C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Combustion reaction
14. 2 KBr + MgCl2 → 2 KCl + MgBr2
Double Replacement reaction
15. 2 HNO3 + Ba(OH)2 → Ba(NO3)2 + 2 H2O
Double Replacement reaction
16. C5H12 + 8 O2 → 5 CO2 + 6 H2O
Combustion reaction
17. 4 Al + 3 O2 → 2 Al2O3
Synthesis reaction
18. Fe2O3 + 2 Al → 2 Fe + Al2O3
Single Replacement reaction
Learn more about Chemical reactions, here:
https://brainly.com/question/29762834
#SPJ1
2. What is the boiling point of a solution of 0.10 mole of glucose in 200 mL of water? (Kp = 0.512°C/m)
O 100.13°C
100.06°C
100.5°C
100.26°C
Answers
Answer:
The Answer is 100.06c
Explanation:
GradPoint:)
Reduction can be described as:A. the gain of electrons.B. None of theseC. the gain of an OH group.D. the loss of an OH group.
Answers
Answer
A. the gain of electrons.
Explanation
Oxidation and reduction are complementary reactions. Oxidation is the process involving with loss of electrons or an increase in oxidation state by a molecule, atom, or ion while reduction is the process of gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.
Hence, the correct answer to your question is option A. the gain of electrons.
Name of element or compoundFormula of element or compound CoCarbon Iron Au KCl CS2Silicon
Answers
Answer:
• Formula: ,Co,, Name: ,Cobalt,.
,• Formula: ,C,, Name: ,Carbon,.
,• Formula: ,Fe,, Name:, Iron,.
,• Formula: ,Au,, Name: ,Gold,.
,• Formula: ,KCl,, Name: ,Potassium chloride,.
,• Formula: ,CS2,, Name: ,Carbon disulfide,.
,• Formula: ,Si,, Name: ,Silicon,.
Explanation:
• Co is an element.
,• Carbon is an element.
,• Iron is an element.
,• Au is an element.
,• KCl is a compound with K and Cl. The name has the positive part first (Potassium) and the second part has the name of the negative part (chloride).
,• CS2 is a compound with C and 2 S. The name has the number of each element, carbon is 1 so it is just Carbon, and there are 2 sulfurs (disulfide).
,• Silicon is an element.
what is the empirical formula for potassium oxide?
Answers
Answer:
Potassium oxide is a metal oxide with formula K2O. It is a potassium salt and a metal oxide.
Explanation: