163 ml of hydrogen gas, measured at 23°C and 1.5 atm pressure, and 249 ml of nitrogen gas,
measured at 23°C and 2.6 atm pressure, were forced into the 163 ml container at 23°C, what
would be the pressure (in atm) of the mixture of gases now in the 163 mil container? Enter to 2
decimal places.
Answers
The total pressure is 3.9 atm
What is the ideal gas equation?We would have to use the idea gas equation in each case;
Number of moles of the hydrogen = 1.5 * 0.163/0.082 * 296
= 0.24/24.3
= 0.0099 moles
Number of moles of nitrogen = 2.6 * 0.163/0.082 * 296
n = 0.017 moles
Now we know from the Dalton's law that when we mix the gases that the total pressure is the pressure in each of the vessels summed up.
Hence the total pressure is; 1.6 + 2.3 = 3.9 atm
It would have a total pressure of about 3.9 atm.
Learn more about pressure:https://brainly.com/question/356658?
#SPJ1
Related Questions
Sort the resources into the correct categories.
are replaced by natural processes
Renewable Resources
Nonrenewable Resources
cannot be replaced in a short time
are used more quickly than replaced
have fixed amounts
are considered unlimited
are replaced faster than used
Intro
✓ Done
Answers
Answer:
Renewable Resources: are considered unlimited, are replaced faster than used.
Nonrenewable Resources: are used more quickly than replaced, have fixed amounts, cannot be replaced in a short time.
Explanation:
Renewable resources are natural resources that are able to naturally regenerate themselves, hence, they are considered to be unlimited. They are usually replaced faster than they are used because they have a short regeneration time. A good example is the solar energy.
Nonrenewable resources are those natural resources that cannot naturally regenerate and when they do, it takes a very long time (usually millions of years). They are therefore used at a much faster rate than they are being replaced and their natural deposits are more or less fixed due to the long regeneration time. A good example is the crude oil deposit.
Hence:
Renewable Resources: are considered unlimited, are replaced faster than used.
Nonrenewable Resources: are used more quickly than replaced, have fixed amounts, cannot be replaced in a short time.
Answer: !
Explanation:

How many molecules are in 85 grams of Silver?
Answers
Answer:
3.0 × 10
\(3.0 \times {10}^{23} \)
molecules
Explanation:
there are many molecules
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
Find the mass of 2 moles of carbon tetrachloride
Answers
Answer:
The mass of 2 moles of carbon tetrachloride is
307.646 grams
Explanation:
The chemical formula for Carbon tetrachloride is \(\ce{CCl_4}\). It contains 1 carbon atom and 4 chlorine atoms.
Carbon tetrachloride is formed due to the covalent bond between one carbon atom with four chlorine atoms.
In order to find the mass of 2 moles of \(\ce{CCl_4}\) we need to determine the molar mass.
The molar mass of carbon is 12.011 g/mol.
The molar mass of chlorine is 35.453 g/mol.
As stated before we have 1 carbon atom and 4 chlorine atoms. So the molar mass can be evaluated by
\(\left(1*12.011\right)+\left(35.453*4\right)=153.823\)
The molar mass of \(\ce{CCl_4}\) is 153.823 g/mol.
You can multiply that by 2 to get the mass in grams of 2 moles of carbon tetrachloride.
\(2*153.823 =307.646\)
Learn more about covalent bonds here
https://brainly.com/question/28808905
What changes sodium pellets to liquid
Answers
Answer:
when placed in water, a sodium pellet catches on fire as hydrogen gas is liberated and sodium hydroxide forms. chemical change = fire is a sign of chemical reaction.
Explanation:
When placed in water the sodium pellets catch the fire and liberate the hydrogen gas. On mixing with water solid sodium forms a colorless basic solution.
What are the properties of sodium?Sodium is a soft metal. It is a very reactive element with a low melting point. Sodium reacts very quickly with water, snow, and ice to produce sodium hydroxide and hydrogen. It is an alkali metal and the sixth most abundant metal on earth. It has a silvery white color.
It has a strong metallic luster. On reacting with oxygen it produces sodium oxide which on reacting with the water produces sodium hydroxide.
It is used to improve the structure of certain alloys and soaps. It is also used in the purification of metals. Sodium is also present in sodium chloride, an important compound found in the environment.
To learn more about sodium, refer to the link:
https://brainly.com/question/29327783
#SPJ2
HELPPPPPPP PLZZZ
An apple is growing in the top branches of an apple tree. The stem breaks and the apple falls, moving faster and faster until it hits the ground.
Where is the energy to increase the speed of the apple coming from?
Energy is being created as the apple falls
Energy is not related to the speed of an object
The potential energy the apple had due to its height is converted to kinetic energy
The force from the wind that broke the stem is also pushing the apple down
Answers
Answer:
The potential energy the apple had due to its height is converted to kinetic energy
Explanation:
When an apple falls from the tree to the ground, its energy of position stored as gravitational potential energy is converted to kinetic energy, the energy of motion, as it falls. When the apple hits the ground, kinetic energy is transformed into heat energy.
The correct IUPAC name for the structure shown is
A)
ethylmethylamine.
B)
methylamine.
C)
ethylamine.
D)
ethylmethylhydridoamine.

Answers
Answer:
A
Explanation:
it has a methyl group, ethyl group and amine group
The correct IUPAC name for the structure shown in the provided image is "ethylamine." The structure consists of a central nitrogen atom bonded to two carbon atoms.
The correct IUPAC name for the structure shown in the provided image is "ethylamine." The structure consists of a central nitrogen atom bonded to two carbon atoms. According to the IUPAC naming rules, the longest carbon chain is selected as the parent chain, which in this case consists of two carbon atoms. The substituent attached to the parent chain is an ethyl group, denoted as "C2H5". The amine functional group, which consists of the nitrogen atom, is named as "amine". Since there is only one amine group attached to the carbon chain, it is referred to as "ethylamine." Therefore, option C) "ethylamine" is the correct IUPAC name for the given structure.
For more question on IUPAC
https://brainly.com/question/28872356
#SPJ11
balance the chemical reaction ___ Al + ___ FeO → ___ Al2O3 + ___ Fe
Answers
Answer:
2Al+3FeO→Al2O3+3Fe
Explanation:
Basically, to get this answer, you need to balance the amount of Aluminum to the ammount on the other side which is 2 so you need 2 Al to balance the reaction correct, next you move on to the amount of Oxygen in the reaction, there are three Oxygen’s on the right and one on the left, so you need 3 FeO in order for the Oxygens to be balanced. Now that the Iron is unbalanced on both sides, you need 3 Fe (on the right) in order for the equation to be balanced.
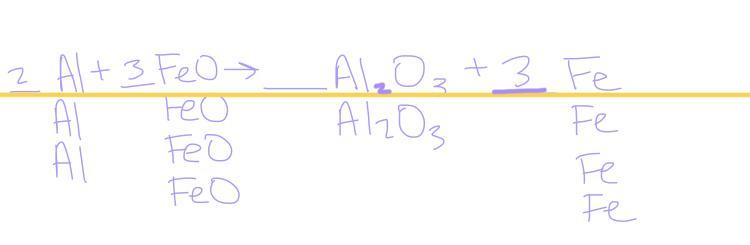
The balanced chemical reaction is \(2Al+3FeO\) → \(Al_2O_3+3Fe\)
What is a balanced chemical reaction?A balanced chemical equation occurs when the number of atoms involved on the reactants side is equal to the number of atoms on the products side.
To balance a reaction means to make the number of atoms the same on both the reactants and products sides. To do so, coefficients need to be added to the chemical equation.
Hence, the balanced the chemical reaction is \(2Al+3FeO\) → \(Al_2O_3+3Fe\).
Learn more about the balanced chemical reaction here:
https://brainly.com/question/15196081
#SPJ2
Which of the following mixtures cannot be separated by using both particle size and color as observable properties?
(A) Nickels and pennies
(B)Sand and rocks
(C) Stick and stones
(D)Salt and beans
Answers
Answer:
(A) Nickels and pennies
What else should the second group of researchers measure to test the hypothesis
Answers
Answer:
Testing of thesis testing is used to determine the plausibility of a thesis.
The test assess the plausibility of the thesis of a sample data.
It occurs through different way, the first step is used to test the state the two suppositions.
In the coming step evaluation of data, third step include carry out plan and dissect the data.
The final step is to dissect the results of the data, it can moreover accept the thesis and reject the null thesis.
Learn further about thesis, then
brainly.com/question/17173491?
How do I balance Ag(s) + H2S(g) + 02(g) arrow Ag2(S(s) + H2O(l)
Answers
Answer:
4 Ag(s) + 2 H2S(g) + O2 === 2 Ag2S(s) + 2H2O
How does a lithium cation compare to a lithium atom?
The cation is larger.
The cation has the same radius.
The cation is smaller.
The cation has the same charge.
Answers
The cation is smaller.
A lithium atom is said to be oxidized when it loses an electron and becomes a positive ion. When lithium forms chemical compounds with other elements, it loses an electron from each lithium atom and becomes a Li+ cation. These are then attracted to the negatively charged anions of ionic compounds.
When an atom loses an electron, it forms a positive ion. The number of protons in a positive ion is greater than the number of electrons. Therefore, the attractive force between the electron and the nucleus increases, reducing the atomic radius. Therefore, the size of atoms is larger than that of cations. A cation is always smaller than its parent atom because its nuclear charge remains the same and it has fewer electrons.
Learn more about A lithium atom here:-https://brainly.com/question/3902528
#SPJ1
(C) The lithium cation is smaller than the lithium atom.
The electronic configuration of the Lithium atom is given as:
1s² 2s¹ and the electronic configuration of Lithium cation is 1s².
The Lithium Cation is smaller because,
Less Energy Levels: As shown in electrical configurations, the Li atom's valence shell is 2 while the Li cations is 1, resulting in a closer spacing between the valence electrons and nucleus and smaller size.
Less Shielding Effect: The shielding effect is the protection provided by the core electrons to the valence electron from the nucleus pull. Since there are no core electrons in a cation of lithium, the electrons are more drawn to the protons, which causes them to be tiny.
More nuclear charge: The Li atom has three protons and three electrons. As a result, the game is slightly balanced because the three protons attract the three electrons. However, as the tone electron in the Li Cation is removed, the proton side becomes stronger and exerts more force on the electrons, bringing them closer to the nucleus and reducing their size.
Hence, the cation is smaller.
Learn more about cation here:
https://brainly.com/question/14309645
#SPJ1
Help me plssssssssss

Answers
Answer:
The answer for this selection is 4
Explanation:
calculate the mol fraction of ethanol and water in a sample of rectified spirit which contains 95% of ethanol by mass
Answers
Answer:
We are given that there is 95% ethanol by mass in rectified spirit
so, we can say that in a 100g sample, we have 95 grams of ethanol and 5 grams of water
we will find the number of moles of ethanol and water in 100g solution of rectified spirit and use that to calculate the mole fraction
Moles of Ethanol:
Molar mass of ethanol = 46 grams / mol
Number of moles = Given mass / molar mass
Number of moles = 95 / 46
Moles of Ethanol = 2 moles (approx)
Moles of Water:
Molar mass of water = 18 grams per mol
Number of moles = Given mass / molar mass
Moles of water = 5 / 18
Moles of water = 0.28 moles (approx)
Mole Fractions:
Mole fraction of a specific compound is the number of moles of that compound divided by the total number of moles in the solution
Mole fraction of Ethanol:
Moles of ethanol / (moles of ethanol + moles of water)
2 / (2 + 0.28)
2 / (2.28) = 0.9 (approx)
Mole fraction of Water:
Moles of water / (Moles of ethanol + moles of water)
0.28 / (2 + 0.28)
0.28 / (2.28) = 0.1 (approx)
Can you help me solve number 4?

Answers
The pressure of the gas in the flask, given that the temperature is reduced to -15 is 0.38 mmHg
How do i determine the pressure of the gas?From the question given above, the following data were obtained:
Initial pressure (P₁) = 338 mmHgInitial temperature (T₁) = 72 °C = 72 + 273 = 345 KInitial volume (V₁) = 0.225 LNew volume (V₂) = 150 LNew temperature (T₂) = -15 °C = -15 + 273 = 258 KNew pressure (P₂) = ?The new pressure of the gas can be obtained by using the combined gas equation as shown below:
P₁V₁ / T₁ = P₂V₂ / T₂
(0.225 × 338) / 345 = (P₂ × 150) / 258
Cross multiply
345 × 150 × P₂ = 0.225 × 338 × 258
Divide both sides by (345 × 150)
P₂ = (0.225 × 338 × 258) / (345 × 150)
P₂ = 0.38 mmHg
Thus, we can conclude the pressure of the gas is 0.38 mmHg
Learn more about pressure:
https://brainly.com/question/15343985
#SPJ1
The heat capacity of copper metal is 0.38 J/goC. Assume you had a 75 g cube of copper at 25.0oC. What would the final temperature of the copper be (in oC) if it absorbed 150 J of heat?
Answers
The final temperature of the copper would be 30.26°C if it absorbed 150 J of heat.
To solve this problem, we can use the formula:
q = m * C * deltaT
where q is the heat absorbed by the copper, m is the mass of the copper, C is the heat capacity of copper, and deltaT is the change in temperature.
Rearranging the formula, we get:
deltaT = q / (m * C)
Substituting the given values, we get:
deltaT = 150 J / (75 g * 0.38 J/g°C) = 5.26 °C
Therefore, the final temperature of the copper will be:
25.0°C + 5.26°C = 30.26 °C
Learn more about heat capacity, here:
https://brainly.com/question/28302909
#SPJ1
A 10.0 cm3 sample of copper has a mass of 89.6 g. What is the density of copper
Answers
Answer:
Density = 8.96 g/cm³Explanation:
The density of a substance can be found by using the formula
\(Density = \frac{mass}{volume} \)
From the question
mass of copper = 89.6 g
volume = 10 cm³
Substitute the values into the above formula and solve
That's
\(Density = \frac{89.6}{10} \)
We have the final answer as
Density = 8.96 g/cm³Hope this helps you
A burning match will burn more vigorously in pure oxygen than in air because _________ . Select one: a. oxygen is a catalyst for combustion b. nitrogen is a reactant in combustion and its low concentration in pure oxygen catalyzes the combustion c. oxygen is a product of combustion d. nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature e. oxygen is a reactant in combustion and pure oxygen increases the reactant concentration
Answers
Answer:
e. oxygen is a reactant in combustion and pure oxygen increases the reactant concentration
Explanation:
The reaction of a burning match is combustion. In this combustion, the organic components of the match (such as cellulose, C₆H₁₀O₅) react with oxygen, producing water and carbon dioxide:
C₆H₁₀O₅(s) + 6O₂(g) → 5H₂O(g) + 6CO₂(g)Seeing as oxygen is a reactant and not a catalyst nor product, and that nitrogen plays no part in the reaction, the only correct answer is option e.
What would change oxygen into another isotope
Answers
Answer:
Adding Neutrons
General Formulas and Concepts:
Chemistry - Atomic Structure
Atoms CompositionNucleus - Protons, NeutronsElectronsExplanation:
We know that adding Protons to any atom will change its chemical properties and make it a different element.
We also know that adding Electrons to any atom will simply only change its overall charge.
Isotopes are formed by adding neutrons to the nucleus. It will be the same element but it would have a different mass and amount of neutrons.
If ammonia is produced at a certain time, t, at a rate of 0.397 M/sec, what is the rate for each of the reactants
Answers
When ammonia is produced at a rate of 0.397 M/s, nitrogen is consumed at a rate of 0.199 M/s, and hydrogen is consumed at a rate of 0.596 M/s.
What is the rate of reaction?The rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time.
Step 1: Write the balanced equation for the synthesis of ammonia.N₂ + 3 H₂ ⇄ 2 NH₃
Step 2: Calculate the rate of consumption of nitrogen.The molar ratio of N₂ to NH₃ is 1:2.
0.397 mol NH₃/L.s × (1 mol N₂/2 mol NH₃) = 0.199 mol N₂/L.s
Step 3: Calculate the rate of consumption of hydrogen.The molar ratio of H₂ to NH₃ is 3:2.
0.397 mol NH₃/L.s × (3 mol H₂/2 mol NH₃) = 0.596 mol H₂/L.s
When ammonia is produced at a rate of 0.397 M/s, nitrogen is consumed at a rate of 0.199 M/s, and hydrogen is consumed at a rate of 0.596 M/s.
Learn more about rates of reaction here: https://brainly.com/question/24795637
How then do we have two alleles for a trait?
Answers
Answer:
one from each parent
Explanation:
Calculate the theoretical percentage of water for the following hydrates.
(a) manganese(II) monohydrate, MnSO4 H2O
(b) manganese(II) tetrahydrate, MnSO4 4H2O
Answers
Answer:
(a) \(\% H_2O=10.65\%\)
(b) \(\% H_2O=32.2\)
Explanation:
Hello.
For this questions we must consider the ratio of the molar mass of water to hydrated compound molar mass as shown below:
(a) In this case, we can consider that inside the manganese (II) sulfate monohydrate, whose molar mass is 169.02 g/mol, there is one water molecule that has a molar mass of 18 g/mol, for which the theoretical percentage of water is:
\(\% H_2O=\frac{18g/mol}{169.0g/mol} *100\%\\\\\% H_2O=10.65\%\)
(b) In this case, we can consider that inside the manganese (II) sulfate tetrahydrate, whose molar mass is 223.1 g/mol, there are four water molecules that have a molar mass of 4*18 g/mol, for which the theoretical percentage of water is:
\(\% H_2O=\frac{4*18g/mol}{223.1g/mol} *100\%\\\\\% H_2O=32.27\%\)
Best regards.
12.24 cm (wavelength of a microwave oven)
Answers
The question is incomplete, however, 0.245 x 10^10 s^ -1 is the frequency of 12.24 cm (wavelength of a microwave oven).
we have given, λ= 12.24 cm, in the statement,
ν = c / λ where c is the velocity of light , i.e 3 x 10^10 cm/s
So, putting the value of c and λ= 12.24 cm,
we get,
ν = 3x 10^10 cm s^-1 / 12.24 cm
= 0.245 x 10^10 s^-1
Frequency is the quantity of full oscillations generated by a wave particle in a unit of time.When a wave travels from one place to another, its frequency describes how frequently a medium particle vibrates. The frequency of a wave may be calculated mathematically by counting how many full oscillations are produced during its propagation in one unit of time. The number of waves passing a given location in a unit of time may thus be used to characterize it.To know more about frequency visit : https://brainly.com/question/14316711
#SPJ1
Select the correct answer from
each drop-down menu.
What causes O atoms to form covalent bonds?
In a covalent bond, two atoms are held together by the attraction between
bonds that an atom can form depends on the number of
The number of covalent
in the atom.
Answers
The oxygen atoms form covalent bonds as they have same value of electronegativity.
What is covalent bond?
Covalent bond is defined as a type of bond which is formed by the mutual sharing of electrons to form electron pairs between the two atoms.These electron pairs are called as bonding pairs or shared pair of electrons.
Due to the sharing of valence electrons , the atoms are able to achieve a stable electronic configuration . Covalent bonding involves many types of interactions like σ bonding,π bonding ,metal-to-metal bonding ,etc.
Sigma bonds are the strongest covalent bonds while the pi bonds are weaker covalent bonds .Covalent bonds are affected by electronegativities of the atoms present in the molecules.
Learn more about covalent bond,here:
https://brainly.com/question/19382448
#SPJ9
Complete combustion of 8.90 g of a hydrocarbon produced 27.3 g of CO₂ and 13.0 g of H₂O. What is the empirical formula for
the hydrocarbon? Insert subscripts as necessary.
Answers
Empirical formula is C4H7.
Empirical formula- A formula that lists the relative amounts of the constituent components in a compound without specifying the number or configuration of atoms.
When a hydrocarbon (CxHy) burns, CO2 and H2O are produced.
(Note: It mentions that a hydrocarbon is burned, which suggests that oxygen isn't present.)
moles of C in the compound: 44 g x 1 mol C/mole and 27.8 g CO2 x 1 mol CO2. = 0.632 moles of carbon
9.96 g H2O x 1 mol H2O/18 g x 2 mol H/mol H2O Equals 1.11 moles of H in the molecule.
We can calculate the mass and verify that it adds up to 8.70 g to rule out the presence of oxygen in the original molecule.
mass C=0.632 mol C times 12 g/mol equals 7.58 g C
H mass equals 1.11 mol H = 1.11 g H x 1 g/mol
Total mass Equals 8.69 g.
We may divide both by the lowest value (0.632), which will give us the lowest whole number of moles.
0.632/0.632 = 1.0 moles C
H = 1.11/0.632 = 1.75 moles.
Now we can multiply them by 4 to get H's whole number, which is-
C = 4 moles.
H = 7 moles
∴Empirical equation: C4H7
To learn more about empirical formula refer - https://brainly.com/question/1603500
#SPJ9
ethane (c2h6) is cheap and readily available, but not particularly reactive. ethane can be dehydrogenated to ethylene (c2h4), which is often used as a raw material for polymer manufacturing.
Answers
Ethane can be dehydrogenated to ethylene, which is often used as a raw material for polymer manufacturing.
The process of dehydrogenation involves removing hydrogen from an organic substance to create a new chemical (e.g., to convert saturated into unsaturated compounds).
Alcohols are dehydrogenated to create aldehydes and ketones. Since it guarantees great specificity and transition at a lower temperature range of 600-700 C, non-oxidative dehydrogenation of ethane (NODHE) to ethylene poses an entirely different approach to steam cracking (SC), the prevailing standard technique for mass producing ethylene.
This reduces energy consumption and carbon emissions. The reactions are:
\(C_{2}H_{6} \rightarrow C_{2}H_{4} + H_{2}\)
Learn more about Dehydrogenation
brainly.com/question/25500594
#SPJ4
HELP FAST PLZ!!!!! Which phase change allows a substance to transform from a liquid to a
gas?
melting
Ofreezing
O ionization
condensation
deionization
O
evaporation
sublimation
Answers
Answer:evaporation
Explanation:
Answer:
Pretty sure its evaporation, if its not I'm very sorry.
If the number of equally likely sample outcomes of a single ASAP! stage of an experiment is 4, what is the total number of elements in the sample
space if the experiment has 3 stages?
OA. 16
OB. 12
OC. 64
OD. 81
Answers
Answer:
C. 64
Explanation:
Given:
The number of equally likely sample outcomes of a single stage = 4
Unknown:
Total number of elements if there are three stages= ?
Solution;
Probability is the likelihood of an event to occur.
In this problem;
For a single stage, the likely outcome is 4
So therefore;
For the 3 stages 4 x 4 x 4 = 64
Therefore, the total number of elements in the sample space of an experiment with 3 stages is 64
is oxygen an atom? Please help i have done this project but i think i did it wrong
Answers
Answer: oxygen is not a atom Oxygen is the chemical element with the symbol O and atomic number 8
Explanation:
what happens when an electrolyte, NaCl is added to hydrate ferric oxide solution
Answers
Answer:
hydrated ferric oxide is ferric hydoxide sol and is positively charged. When aqueous solution of NaCl is added to it,the Cl- ions neutralise the positive charge on the sol particles. In the absence of charge, brown precipitate is formes due to colloids can be coagulation of particles.Nov 11, 2020
Explanation: hope this help