1. What mass of CO₂ can be produced from 25.0 grams of CaCO3, given the decomposition
reaction below? CaCo3=CaO+CO2
Answers
11 grams of CO₂ can be produced from 25.0 grams of CaCO₃, This is decomposition reaction of CaCO₃ taken out by mole concept.
What are decomposition reaction ?A decomposition reaction occurs when a chemical is broken down into two or more simpler components. A decomposition reaction has the generic form: AB = A+B. The majority of decomposition reactions require an energy input in the form of heat, light, or electricity. When one reactant breaks down into two or more products, this is referred to as a decomposition reaction. This is expressed by the following generic equation: AB A + B. • Decomposition processes include the conversion of hydrogen peroxide to water and oxygen, as well as the conversion of water to hydrogen and oxygen.
To know more about decomposition reaction , visit ;
brainly.com/question/16987748
#SPJ1
Related Questions
Choose the best answer.
What is the sum?
2/3+7/15
O 14/15
O1/2
O1 2/15
O3/5
Answers
Answer:
1 2/15
Explanation:
2/3 + 7/15 = 17/15 =1 2/15
Iron reacts with oxygen to form rust according to the equation: 2 Fe + 3 O2 --> 2 Fe2O3
If you react 60,5 moles of oxygen gas, how many moles of rust can you form?
Answers
Answer: 40.3
Explanation:
In the reaction, we see that for every 3 moles of oxygen gas consumed, 2 moles of rust are formed.
So this means that if 60.5 moles of oxygen gas are consumed, then (60.5/3)(2)=40.3 moles of rust can be formed.
Mrs. Keep burns a walnut under a beaker of water. The beaker contains 100 g of water which warms from 25oC to 30oC. Assuming that all the heat from the burning walnut goes into the water and none of the heat is lost to the air or the beaker, how many calories are in the walnut?
a 2100 calories
b 10,500 calories
c not enough information is given
d 500 calories
Answers
The amount of heat gained by the water is 500 calories. Thus, option D is correct.
Given:
Mass of water (m) = 100 g
Change in temperature (ΔT) = 30°C - 25°C = 5°C
The specific heat capacity of water (c) is approximately 1 calorie/gram°C.
Now, the amount of heat gained by the water,
Q = mcΔT
Where:
Q is the heat gained or lost by the substance
m is the mass of the substance
c is the specific heat capacity of the substance
ΔT is the change in temperature
Plugging in the values into the formula:
Q = 100 × 1 × 5
Q = 500 calories
Therefore, the amount of heat gained by the water is 500 calories.
Learn more about heat, here:
https://brainly.com/question/31608647
#SPJ1
What is required for two atoms to share electrons equally in a chemical bond?
A. The two atoms must have the same number of valence electrons.
B. The two atoms must both be nonmetals.
C. The two atoms must have equal and opposite charges.
D. The two atoms must be of the same element.
Answers
Answer:
D
Explanation:
The two atoms must be of the same element in order for two atoms to share electrons equally in a chemical bond.
What is an element?
An element is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
The number of protons in the nucleus is the defining property of an element and is related to the atomic number.All atoms with same atomic number are atoms of same element.
Learn more about element,here:
https://brainly.com/question/14347616
#SPJ5
During the process of condensation, water vapor
A. releases 334 J/g of heat energy
B. releases 2260 J/g of heat energy
C. gains 334 J/g of heat energy
D. gains 2260 J/g of heat energy
Answers
Answer:
A
Explanation:
it releases 4
334 J/g of heat energy
hope its correct
What is the oxidizing agent in the reaction Fe+AgNO3-->Fe(NO3)3+AG?
A.AgNO3+
B. fe
C.Ag
D. Fe(NO3)3
Answers
The oxidizing agent in the reaction Fe + \(AgNO_3\)→ \(Fe(NO_3)_3\) + Ag is option a \(AgNO_3.\)
A redox reaction is one in which the oxidation states of two species undergo changes. Iron is oxidized in the reaction, while silver nitrate is reduced. One of the reactants is being reduced, whereas the other is being oxidized.The oxidizing agent is the species that is being reduced, and it is the species that accepts electrons.
Fe is being oxidized in this reaction. Therefore, it cannot be the oxidizing agent, nor can\(Fe(NO_3)_3\). In contrast, \(AgNO_3.\) is being reduced, which means it is accepting electrons. This is why\(AgNO_3.\) is the oxidizing agent.The correct answer is option a.
Know more about oxidizing agent here:
https://brainly.com/question/14041413
#SPJ8
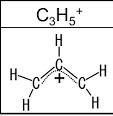
What can you conclude about the symmetry of the C3H5 ion as it actually exists, as regards the bond lengths and the distribution of the positive charge
Answers
In the allyl cation, all the bond lengths are equal and the positive charge is delocalized over all the three carbon atoms.
The allyl cation has the formula C2H5+. In the allyl cation, the positive charge is delocalized over the three carbon atoms as shown in the image attached. Hence, relevant resonance structures can be proposed for the allyl cation.
The bond lengths in the allyl cation are intermediate between the bond lengths of double bonds and single bonds. All the bond lengths are equal.
Learn more: https://brainly.com/question/12108425
4 point
13.
A lynx, Lynx canadensis, has a short tail with
a black tip running all the way around the
tail. It also has highly visible tufts of hair on
the ears. A bobcat, Lynx rufus, has a short
tail with black only on top of the tail's tip.
It also has inconspicuous ear tufts. From
the descriptions and scientific names of both
animals you can conclude that
A.
the lynx and bobcat are the same
species.
B.
"lynx" and "bobcat" are two names for the
same animal.
C.
the lynx and bobcat are the same genus.
D.
the lynx and bobcat are not from the
same phyl
Answers
The descriptions and scientific names of both animals, it can be concluded that the physical lynx and bobcat are not the same species as they have different scientific names - Lynx canadensis and Lynx rufus.
The differences in their physical characteristics, such as the pattern and visibility of their tail and ear tufts, further support the idea that they are different species. However, they are in the same genus, which is Lynx. Therefore, option C is the correct answer. It is important to note that even though they are different species, they share similar characteristics and are often confused with each other. Both the lynx and bobcat are medium-sized wild cats that are native to North America and are known for their elusive nature and hunting abilities.
learn more about physical here.
https://brainly.com/question/13237992
#SPJ11
Calcium carbonate reacts with sulfur dioxide and oxygen gases to produce calcium sulfate and carbon dioxide. calculate the number of tons of caco3 needed to react completely with 1.90 tons of so2.
Answers
In the given reaction of Sulfur dioxide and calcium carbonate, for complete reaction, 1.90 tons of sulfur dioxide would require 2.9 tons of Calcium carbonate.
Balancing a chemical equation follows the law of mass conservation. The quantity of an element in the product and in reactants should be the same. Balanced equation of the reaction:
2CaCO3 + 2SO2 + O2 → 2CaSO4 + 2CO2
Two moles of calcium carbonate react with two moles of sulfur dioxide and give two moles each of calcium sulfate and carbon dioxide.
One ton is equal to a thousand kilograms and one kilogram is equal to a thousand grams. Which means one ton is equal to 10^6 grams.
The moles of a compound are obtained using the following formula:
Moles = given mass in grams/ molar mass
Tons of SO2 = 1.90 tons
Molecular mass of SO2 = 64
Moles of SO2 = (1.90 x 10^6) /64 = 0.029 x 10^6
Now, 0.029 x 10^6 moles of SO2 would completely react with 0.029 x 10^6 moles of CaCO3.
The molar mass of CaCO3 = 40+12+16+16+16 =100
Grams in 2.9 x 10^6 moles of CaCO3 = moles x molecular mass
Grams in 2.9 x 10^6 moles of CaCO3= 100 x 0.029 x 10^6
Grams in 2.9 x 10^6 moles of CaCO3 = 2.9 x 10^6
In conclusion, 1.90 tons of sulfur dioxide would require 2.9 tons of Calcium carbonate to react completely.
Learn more about here sulfur:
https://brainly.com/question/31610833
#SPJ12
Table salt (sodium chloride) is 39.1% sodium by mass. How many grams of table salt contain 72.0 g of sodium?
a) 28.2 g
b) 72.0 g
c) 102 g
d) 2820 g
e) 1.84 x 102 g
Answers
The answer is e) 1.84 x 102 g. This means that 184.16 grams of table salt are needed to provide 72.0 grams of sodium.
Table salt, also known as sodium chloride, is a common chemical compound used in various applications. It is composed of two elements: sodium and chlorine, with sodium accounting for 39.1% of the compound's mass. In this problem, we are asked to determine how many grams of table salt are needed to provide 72.0 grams of sodium.
To solve this problem, we can use the information that table salt is 39.1% sodium by mass. This means that in every 100 grams of table salt, 39.1 grams is sodium. We can use this proportion to determine how much table salt is needed to provide 72.0 grams of sodium.
Let x be the amount of table salt (in grams) needed to provide 72.0 grams of sodium. Using the proportion, we can set up the following equation:
39.1/100 = 72.0/x
To solve for x, we can cross-multiply and simplify:
39.1x = 7200
x = 7200/39.1
x ≈ 184.16
Here you can learn more about sodium chloride
https://brainly.com/question/9811771#
#SPJ11
Who thought fire was one of four elements?
O A. Aristotle
O B. Marie Curie
O C. John Dalton
O D. Robert Boyle
Answers
Answer:
D Robert Boyle
Explanation:
Model an oxygen atom and label the parts. Note the type of electric charge for each part. Then complete the sentence that follows. The overall charge of the oxygen atom is _____, because the atom _____.
Answers
Answer:
The overall charge of the oxygen atom is zero, because the atom contain an equal number of electrons and protons.
Explanation:
Pls brainliest
A vehicle is traveling at a speed of 12.5ms-1. Express this speed in light year per year
Answers
A vehicle traveling at a speed of 12.5 m/s is equivalent to 4.171 x 10⁻⁸ light years per year.
What is the speed in light year per year?To convert a speed from meters per second to light years per year, we need to use conversion factors.
1 light year = 9.461 x 10¹⁵ meters
1 year = 31,536,000 seconds
So, to convert 12.5 m/s to light years per year using the conversion factor, 1 year = 31,536,000 seconds:
12.5 m/s = 12.5 x 31,536,000 m/year
12.5 m/s = 3.9468 x 10⁸ m/year
Now, we can convert meters per year to light years per year using the conversion factor 1 light year = 9.461 x 10^15 meters:
3.9468 x 10⁸ m/year = (3.9468 x 10^8 m/year) / (9.461 x 10^15 m/light year)
3.9468 x 10⁸ m/year = 4.171 x 10⁻⁸ light years per year
Learn more about speed in light year per year at: https://brainly.com/question/803764
#SPJ1
In the reaction h ─ h ⟶ h + h, what describes an average energy change of 436 kj/mol? (h2 + 436 kj/mol → 2 h ):
a. the energy will be required as bonds are being broken.
b. the energy will be required as bonds are being formed.
c. the energy will be released as bonds are being broken.
d. the energy will be released as bonds are being formed
Answers
In the reaction H─H ⟶ H + H, with an average energy change of 436 kJ/mol (H2 + 436 kJ/mol → 2 H), the correct description is:
a. The energy will be required as bonds are being broken.
When a chemical reaction involves breaking bonds, energy is typically required to overcome the attractive forces holding the atoms together in the molecule. Breaking a covalent bond requires an input of energy, as the atoms involved need to move apart and overcome their mutual attraction.
In the case of the reaction H─H ⟶ H + H, the hydrogen molecule (H2) is composed of two hydrogen atoms held together by a covalent bond. In order to separate the two hydrogen atoms and form two individual hydrogen atoms, the covalent bond must be broken.
This requires an input of energy to overcome the bond's strength and break the attractive forces between the atoms.
The given average energy change of 436 kJ/mol indicates the amount of energy required to break one mole of hydrogen molecules into individual hydrogen atoms. This energy is needed to disrupt the H─H bond and separate the atoms.
Therefore, the correct description for this reaction is that energy will be required as bonds are being broken.
To learn more about hydrogen, refer below:
https://brainly.com/question/28937951
#SPJ11
What is the molecular weight and the number of equivalents for Ca3(PO4)2
Answers
The molecular weight and the number of equivalents for \(Ca_3(PO_4)^2}\) is 310.18 g/mol and 12 equivalents.
The molecular weight of \(Ca_3(PO_4)^2}\) can be calculated by adding the atomic weights of all the atoms in the compound.
The atomic weights of calcium (Ca), phosphorus (P), and oxygen (O) are:
Ca: 40.08
P: 30.97
O: 15.99
Therefore, the molecular weight of Ca3(PO4)2 can be calculated as follows:
3Ca + 2P + 8O = (3 x 40.08) + (2 x 30.97) + (8 x 15.99) = 310.18 g/mol
The number of equivalents (eq) of \(Ca_3(PO_4)^2}\) depends on the number of reactive ions or groups that the compound can donate or accept. In this \(Ca_3(PO_4)^2}\) contains 3 calcium ions and 2 phosphate ions.
Calcium ions (\(Ca_2\)+) have a valence of 2, meaning they can donate two electrons or accept two electrons. Therefore, each calcium ion has two equivalents.
Phosphate ions (\((PO_4)^2}\)-) have a valence of 3-, meaning they can donate three electrons or accept three electrons. Therefore, each phosphate ion has three equivalents.
To calculate the total number of equivalents in \(Ca_3(PO_4)^2}\), we can multiply the number of ions by their respective equivalents:
3 x 2 equivalents (for calcium ions) + 2 x 3 equivalents (for phosphate ions) = 12 equivalents
Therefore, \(Ca_3(PO_4)^2}\)has a molecular weight of 310.18 g/mol and 12 equivalents.
Know more about molecular weight here:
https://brainly.com/question/24258041
#SPJ11
This substance is water-soluble. A solution of this compound in water would be classified as a(n)___.
Answers
Anything that includes water as a solvent is called an aqueous solution.
Using reliable internet resources, identify ways we use radio waves on Earth.
Answers
Answer:
Ways we use radio waves on Earth are television and FM and AM radio broadcasts, military communications, mobile phones, ham radio, wireless computer networks, and numerous other communications applications.
Explanation:
Ways we use radio waves on Earth are television and FM and AM radio broadcasts, military communications, mobile phones, ham radio, wireless computer networks, and numerous other communications applications.
What are radio waves?An electromagnetic wave of a frequency between about \(10^4\) and \(10^{11}\)or \(10^{12}\) Hz, as used for long-distance communication.
AM and FM Radio Broadcasting which involves transmitting sound to a wide audience.
Radar is a detection system that uses radio waves to get information about objects.
Bluetooth and wireless communication use radio waves to create connections between devices.
Learn more about radio waves here;
https://brainly.com/question/13989450
#SPJ2
What will happen to an object in motion with no unbalanced force acting
on it?
Answers
What does (w/w)% mean?
Answers
Answer: 2% w / w solution means grams of solute is dissolved in 100 grams of solution. ... 5% v / v solution means 5 ml of solute is dissolved 100 ml of solution.
Explanation:
so (w/w)% u would need a number to use it.
Bases are sharp and sweet in taste.
TRUE
FALSE
Answers
Which determines the reactivity of an alkali metal?
its boiling and melting points
the shininess of its surface
the number of protons it has
its ability to lose electrons
Answers
Answer:
its ability to lose electron
The reactivity of an alkali metal is determined by its ability to loose electrons.
The alkali metals are highly electropositive. They easily loose electron to form a univalent positive ion.
This ability to form a univalent positive ion increases down the group hence cesium is the most electropositive element in nature.
Learn more: https://brainly.com/question/18153051
which choice shows the transition state for the given sn2 reaction?
Answers
The correct option is B. In the SN2 reaction, the mechanism will be simultaneous bond-making and bond-breaking occurs.
A reaction refers to the process in which two or more molecules interact and undergo chemical changes to form different molecules. During a chemical reaction, the molecules involved can break apart, rearrange, and form new chemical bonds. This process involves the transfer or sharing of electrons between the reacting molecules.
Reactions can be classified into different types based on their characteristics, such as exothermic or endothermic, reversible or irreversible, and acid-base, oxidation-reduction, or precipitation reactions. In addition, reactions can be influenced by various factors such as temperature, pressure, concentration, and catalysts.
Understanding chemical reactions are important for many applications in various fields, such as in the design of new drugs, the development of new materials, and the production of chemicals and fuels. Chemists use various tools and techniques to study and analyze chemical reactions, such as spectroscopy, calorimetry, and computational methods.
To learn more about Reaction visit here:
brainly.com/question/17434463
#SPJ4


how do Newton's laws of motion describe when and how objects move?
Answers
What is a solvent? please answer
A. The material that is dissolved
B. The material that increases the speed of the dissolution
C. The material that reduces how much can be dissolved
D. The material that is dissolving another material
WILL MARK BRAINLIEST
Answers
Answer:
D
Explanation:
Solvent is the material that is dissolving another material. Thus, option D is correct.
A solvent is a substance that dissolves another substance to form a solution. The substance that is being dissolved is called the solute, and the substance that is doing the dissolving is called the solvent. The solute is usually present in a smaller amount than the solvent, and it is the solvent that determines the physical properties of the solution, such as its density, viscosity, and boiling point.
For example, when salt is dissolved in water, the salt is the solute and the water is the solvent. The water molecules surround the salt molecules and break them apart, so that the salt ions are free to move around in the solution. The solution is then a homogeneous mixture of salt ions and water molecules.
Thus, option D is correct.
Learn more about solvent on:
https://brainly.com/question/11985826
#SPJ3
What must occur for the enthalpy of solution to be negative?
The process of separating the solvent particles from one another must release energy.
The process of separating the solute particles from one another must release energy.
More energy is absorbed as the solute and solvent mix than is released when the solute particles and the solvent particles separate.
More energy is released as the solute and solvent mix than is absorbed when the solute particles and the solvent particles separate.
Answers
Answer:
d on edge my guys.
Explanation:
Can someone please help me with this?-- 18 pts!
What is true about the latent heat of condensation for a substance?
Select all that apply.
The latent heat of condensation will have the same absolute value as the latent heat of vaporization.
The latent heat of condensation will have the same absolute value as the latent heat of solidification.
The latent heat of condensation is a characteristic property that can be used to identify a substance.
The latent heat of condensation will be negative.
The latent heat of condensation is the same for all substances under the same conditions.
The latent heat of condensation will be positive.
Happy Holidays!!
Answers
Answer:
latent heat of condensation will have the same absolute value as the latent heat of vaporization. The latent heat of condensation will be negative. The latent heat of condensation is a characteristic property that can be used to identify a substance.
Explanation:
Answer:
energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature.
Explanation:
A compound boolean expression created with the _______________ operator is true only if both of its subexpressions are true.
Answers
A compound Boolean expression created with the AND operator is true only if both of its subexpressions are true.
A Boolean expression is an expression in which values are either true or false. It is mostly used in programming languages. True and false are denoted by 1 and 0 respectively.
The basic Boolean operators are AND, OR, and NOT. In OR operation, the value is true if either one or the values are true. Not operator also known as negation converts the true value into false and vice versa.
Boolean algebra is the basic fundamental of digital circuits and programming languages.
If you need to learn more about Boolean algebra, click here.
https://brainly.com/question/26659438?referrer=searchResults
#SPJ4
A sample of hydrogen gas H2 has a volume of 5.0 L and a pressure of 1.0 atm. What is the final pressure in atmospheres if the volume is decreased to 2 L with no change in temperature and amount of gas
Answers
Answer:
2.5 atm
Explanation:
P = Pressure
V = Volume
P1V1 = P2V2
1 x 5 = P2 x 2
5 = P2 x 2
Divide both sides by 2
5/2 = P2 x 2/2
P2 = 2.5
2nd time posting someone please help

Answers
Answer:
Sb: Antimony
Si: 14 (atomic Number)
S: 32.06 u (atomic mass)
Explanation:
Why does less evaporation mean higher temperatures in urban areas?
Answers
Less evaporation means that less of the sun's energy is used to convert water into water vapor, and more of it is used to heat up the surface. In urban areas, there is typically less vegetation and more impervious surfaces (such as concrete and asphalt), which reduces the amount of water that can evaporate.
This means that more of the sun's energy is absorbed by the surface, leading to higher temperatures. Additionally, buildings and other structures in urban areas can trap heat and prevent it from dissipating, further contributing to the urban heat island effect.
In rural areas, vegetation and soil moisture play an important role in regulating temperature through a process called evapotranspiration. Evapotranspiration is the combined process of water evaporation from the soil and plant transpiration. It helps to cool the air by removing heat from the surface through the transfer of water from the surface to the atmosphere.
In contrast, urban areas have a significant amount of impervious surfaces like concrete, asphalt, and buildings, which reduce the amount of vegetation and soil moisture. As a result, urban areas have less evapotranspiration, which means less cooling effect from the evaporation of water. This leads to a higher surface temperature in urban areas.
Furthermore, urban areas have a higher proportion of dark-colored surfaces, such as asphalt and concrete, which absorb more solar radiation than lighter-colored surfaces like vegetation and soil. This is known as the "urban heat island effect," which further contributes to higher temperatures in urban areas.
For more question on evaporation click on
https://brainly.com/question/8944874
#SPJ11