1. Out of a colloid, solution and a suspension:
(a) which one has the smallest particles ?
(b) which one has the largest particles ?
Answers
Answer:
A solution is always transparent, light passes through with no scattering from solute particles which are molecule in size. ... A colloid is intermediate between a solution and a suspension. While a suspension will separate out a colloid will not. Colloids can be distinguished from solutions using the Tyndall effect.
Explanation:
Related Questions
what are items that are ferromagnetic material
Answers
Answer:
I'll give you three examples:
Iron
Cobalt
Nickel
help pls its just one and i am done i dont get it

Answers
Carbon and oxygen combine to produce carbon dioxide. chemical equation
Answers
Answer:
A molecule of the compound carbon dioxide consists of one atom of carbon and two atoms of oxygen, so carbon dioxide is represented by the chemical formula CO2. Q: What is the chemical equation for this reaction? A: The chemical equation is: C + O2 → CO.
Explanation:
carbonate buffers are important in regulating the ph of blood at 7.40. if the carbonic acid concentration in a sample of blood is 0.0013 m, determine the bicarbonate ion concentration required to buffer the ph of blood at ph
Answers
The blood buffer has a pH of 7.4, whereas the blood sample has a carbonic acid content of 0.0013 M.
The chemical compound of hydrogen, carbon, and oxygen is known as carbonic acid (H2CO3).It is produced in minute quantities when water dissolves its anhydride, carbon dioxide (CO2). Two covalent double bonds bind the core carbon atom of carbon dioxide, also referred to as carbonic acid gas, to the two oxygen atoms.
Calculation:Calculating the concentration is as follows:pH = pKₐ + log HCO⁻₃/ H₂CO₃
7.4 = - log(4.3 × 10⁻₇)+ log HCO⁻₃/ 0.0013m
log HCO⁻₃/ 0.0013m = 7.4 - 6.37
log HCO⁻₃/ 0.0013m = 1.03
log HCO⁻₃/ 0.0013m = 10¹°⁰³
HCO⁻₃ = 1.3 ₓ 10⁻².
To know more about carbonic acid visit:-
https://brainly.com/question/28175742
#SPJ4
Draw the geometric, linkage, and ionization isomers for [CoCl5CN][CN].
Answers
Answer:
See explanation
Explanation:
The formation of isomers is common to octahedral complexes. Isomers are different compounds with the same molecular formula but different structural formulas. Isomers have different atom to atom connections. Werner's complexes can display; polymerization, ionization, linkage, geometric and optical isomerism among others.
Isomers of coordination compounds are not easily recognizable and not easily separable in the laboratory.
The geometric, linkage and ionization isomers of the complex given in the question are shown below.
![Draw the geometric, linkage, and ionization isomers for [CoCl5CN][CN].](https://i5t5.c14.e2-1.dev/h-images-qa/answers/attachments/ok1mAyFvX4ygIm8cDuzVUXb20QvQUl12.jpeg)
According to the kinetic-molecular theory, what is one way the three states of matter are all similar?
Answers
The kinetic molecular theory of matter states that:
1. Matter is made up of particles that are constantly moving.
2. A change in phase may occur when the energy of the particles is changed.
3. All particles have energy, but the energy varies depending on the temperature the sample of matter is in. This in turn determines whether the substance exists in the solid, liquid, or gaseous state.
Write a balanced half-reaction for the oxidation of liquid water to gaseous oxygen in basic aqueous solution. be sure to add physical state symbols where appropriate.
Answers
The balanced half-reaction for the oxidation of liquid water to gaseous oxygen in basic aqueous solution is given as ,
\(4OH^{-}\)(aq) ⟶ \(O_{2} (g)\) + \(2H_{2}O\)(l) + \(4e^{-}\)
Steps involved :
The oxidation of liquid water to gaseous oxygen can be represented as
\(H_{2} O(l)\) ⟶ \(O_{2} (g)\)
To balance this, we will first balance the number of oxygen atoms.
\(2H_{2} O(l)\) ⟶ \(O_{2} (g)\)
Now we have to balance the hydrogen atoms. Since the reaction takes place in an basic aqueous medium, hydroxide will be present in the form of its ions, dissolved in the aqueous medium.
\(2H_{2} O(l)\) + \(4OH^{-}\)⟶ \(O_{2} (g)\) + \(4H^{+}\)+ \(4OH^{-}\)
Now the atoms on either side are balanced, but the charges are not. The reactant have a charge of +4 whereas the product is neutral. So, to balance the charge, we will add electrons to the products side, which was expected since, in an oxidation reaction, electrons are released.
\(2H_{2} O(l)\) + \(4OH^{-}\)⟶ \(O_{2} (g)\) + \(4H_{2}O\) + \(4e^{-}\)
\(4OH^{-}\)(aq) ⟶ \(O_{2} (g)\) + \(2H_{2}O\)(l) + \(4e^{-}\)
This is the required balanced half-reaction.
learn about balanced half-reaction
https://brainly.com/question/15166503
#SPJ4
Which sentence is a statement of the law of conservation of mass?
O A. In a chemical reaction, mass remains the same, even though the
types of atoms can change.
B. In a chemical reaction, the mass per volume of the reactants
equals the mass per volume of the products.
C. In a chemical reaction, atoms can be rearranged, but the total
mass of each type of atom never changes.
O D. In a chemical reaction, mass is conserved, but the numbers and
types of atoms are not.
Answers
Answer:
C
Explanation:
The law of conservation of mass states that the added mass of the reactants is equal to the added mass of the products.
A cannot be because it says that for example if you have two hydrogens in the reactants, those two hydrogens transform in for example 2 oxygen and the hydrogen disappear. that's impossible in chemistry.
B. Volume does not directly deal with that law of conservation of mass
C. Is correct, you can rearrange the atoms but if you keep the same amount of mass for each atom and you do not omit any atom, you are respecting the law of conservation of matter.
D.Same explanation for A
PLS HELP MEEEEEEEEEEEEEE WITH SCIENCEEEEEEEEEE

Answers
Answer:
I believe this is a measuring cylinder? and i think the volume is 35cm^3
Explanation:
hope this helps!
A sample of gas at 53.0 oC and 1.19 atm occupies a volume of 2.3 L. What volume would this gas occupy at 107 oC and 0.60 atm?
Answers
Answer:
\(V_2=5.32L\)
Explanation:
Hello there!
In this case, according to the given pressure, temperature and volume, it is possible for us to calculate the final volume via the combined ideal gas law:
\(\frac{P_2V_2}{T_2}=\frac{P_1V_1}{T_1}\)
In such a way, we solve for the final volume, V2, to obtain:
\(V_2=\frac{P_1V_1T_2}{T_1P_2}\)
Then, we plug in the given data to obtain:
\(V_2=\frac{1.19atm*2.3L*380K}{326K*0.60atm}\\\\V_2=5.32L\)
Regards!
How many moles of gas are contained in 22.41 liters at 101.325 kPa and 0ᴼC? (Note: use Ideal Gas Law, PV = nRT) a 2.5 mole b 1.5 mole c 1.0 mole d 2.0 mole
Answers
Answer:
Therefore, 1.00 mole of the gas is present in the container.
Explanation:
The following data were obtained from the question:
Volume (V) = 22.41L
Temperature (T) = 273K
Pressure (P) = 101.325 kPa
Gas constant (R) = 8.31 L.kPa/mol.K.
Number of mole (n) =...?
The number of mole of the gas in the container can obtained by applying the ideal gas equation as illustrated below:
PV = nRT
Divide both side by RT
n = PV /RT
n =101.325 x 22.41 / 8.31 x 273
n = 1.00 mole.
Therefore, 1.00 mole of the gas is present in the container.
Answer:
1 mole of gas is contained in 22.41 liters at 101.325 kPa and 0ᴼC
Explanation:
Ideal gases are a simplification of real gases that is done to study them more easily. It is considered to be formed by point particles, do not interact with each other and move randomly. It is also considered that the molecules of an ideal gas, in themselves, do not occupy any volume.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P*V = n*R*T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas.
In this case:
P= 101.325 kPa= 1 atmV= 22.41 Ln=?R= 0.082 \(\frac{atm*L}{mol*K}\)T= 0°C= 273 °KReplacing:
1 atm*22.41 L=n* 0.082 \(\frac{atm*L}{mol*K}\)*273 K
Solving:
\(n=\frac{1 atm*22.41 L}{0.082\frac{atm*L}{mol*K} *273 K}\)
n=1 mole
1 mole of gas is contained in 22.41 liters at 101.325 kPa and 0ᴼC
what happens to a light as it passes through a blue drink
all colors are reflected by the drink except for blue which is absorbed by the drink
all colors are refracted by the drink except for blue which is reflected through the drink
all colors are transmitted through the drink except for blue which is absorbed by the drink
all colors are absorbed by the drink except for blue which is transmitted through the drink.
Answers
The Statement "All colors are transmitted through the drink except for blue which is absorbed by the drink." is correct on what happens to a light as it passes through a blue drink.
What is light?Light is a form of electromagnetic radiation that is visible to the human eye. It is a type of energy that travels through space in the form of waves and does not require a medium to propagate. Light is produced by the movement of charged particles and travels at a constant speed of about 299,792,458 meters per second in a vacuum.
When light passes through a blue drink, the liquid absorbs the blue portion of the spectrum and allows the other colors to pass through. This is because the blue pigment in the drink selectively absorbs the blue portion of the spectrum, which is why we perceive the liquid as blue. The other colors in the visible spectrum are transmitted through the drink and are not affected.
Learn about light here https://brainly.com/question/166544
#SPJ1
(for a important test!! and use in your own words!!)
In one or two sentences, describe the connection between evaporation and a rainy climate.
Answers
Describe the relationship between evaporation and a rainy climate in one or two sentences.
How does evaporation explain itself?A liquid transforms into a gas during evaporation. It is easy to visualize when raindrops "vanish" from puddles on a hot day or when wet clothing dries in the heat. Instead of really dissipating in these situations, the liquid water is evaporating into a gas known as water vapor. Global evaporation takes place.
Why is it called evaporation?Evaporation occurs on surfaces. Because it happens when molecules with more kinetic energy from the top layer of the liquid escape into the air, evaporation is a surface phenomenon.
To know more about evaporation visit:
https://brainly.com/question/18800215
#SPJ1
Combustion of 1 g of benzoic acid releases 26.42 kJ. Which of the following represents the heat capacity of the calorimeter if the temperature rise is 4.674°C?

Answers
To answer this question, we have to divide the amount of heat released by the change in temperature:
\(\frac{26.42kJ}{4.674\degree C}=5.652kJ/\degree C\)It means that the correct answer is the last choice 5.652kJ/°C.
Why is it difficult for countries to reduce emissions of carbon dioxide
Answers
Answer:
Curbing global carbon dioxide emissions has been a challenge, primarily because they are being driven higher by countries with low per capita emissions.
Explanation:
"just trust me bro"
how much energy is needed to convert 120g of ice at -35°C to steam at 150°C?
Answers
can you balance 12 blocks on the 3x2 platform
Answers
Along the western coast of the United States is Death Valley, one of the hottest places in the world at the height of summertime. However just to the west is the Pacific Ocean. Death Valley runs from north to south between the Amargosa Range on the east and the Panamint Range on the west; the Sylvania Mountains and the Owlshead Mountains form its northern and southern boundaries, respectively. Using your knowledge of weather and climate and the image below, explain how a desert can form so close to an ocean.
Answers
The air is cooled by the currents, which causes it to rise and warm when it crosses land. This warming causes the air to hydrate, then afterward precipitates as the air passes deeper inland.
Can a desert and an ocean coexist?The impacts of grasslands hitting the sea are typically astounding. Namibia and or the Western Sahara constitute the place where the African desert meets north Mediterranean Sea. Moreover, the Sahara extends eastward to the Red Sea. The Atacama Desert and the Pacific Ocean meet strikingly in northern Chile.
Why do deserts surround chilly ocean currents?Cold ocean currents that move near the shore drive coastal deserts to emerge. The air is stabilised by the chilly winds, which also prevent cloud development. It produces a significant amount of fog. A dense blanket of minute water droplets which are too light to disperse as rain makes up a fog.
To know more about hydrate visit:
https://brainly.com/question/11202174
#SPJ1
When an ionic compound is added to water, the will be attracted to the partially positive h atom of water and the will be attracted to the partially negative o atom of water. As the compound dissolves the ions separate from each other, or.
Answers
Answer:
Ionic compounds dissolve in water because the water molecules hydrate the ions.
Explanation:
To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond.
They do this by hydrating the ions.
Water is a polar molecule. It has a permanent dipole.
The
O
atom has a partial negative charge, and the
H
atoms have a partial positive charge.
When an ionic compound is added to water, the anion is attracted to partially positive hydrogen atom of water and cation is attracted to partially negative oxygen atom of water.
What is an ionic compound?Ionic compound or electrovalent compound is a type of compound which is formed between the two elements when there is an exchange of electrons which takes place between the atoms resulting in the formation of ions.
When the atom looses an electron it develops a positive charge and forms an ion called the cation while the other atom gains the electron and develops a negative charge and forms an ion called the anion.
As the two atoms are oppositely charged they attract each other which results in the formation of a bond called the ionic bond.
Learn more about ionic compounds,here:
https://brainly.com/question/9167977
#SPJ2
The formation of a peptide bond between two amino acids is an example of a(n) ______________reaction.A) cleavageB) condensationC) group transferD) isomerizationE) oxidation reduction
Answers
The formation of a peptide bond between two amino acids is an example of a condensation reaction.
The formation of a peptide bond between two amino acids is an example of a condensation reaction. In a condensation reaction, two molecules join together to form a larger molecule, and a smaller molecule, usually water, is released as a byproduct. In the case of a peptide bond formation, the carboxyl group of one amino acid reacts with the amino group of another amino acid, releasing water and forming a peptide bond. So, the correct answer is B) condensation.
To know more about Condensation Reaction:
https://brainly.com/question/30553575
#SPJ11
what is the oxygen isotope with 8 neutrons
Answers
Answer:
Oxygen-16
Explanation:
Question 4 of 33
C
Q-Science-Gr7-SQSA1-CBT (2020-21)
Question: 1-4
Dead sean animals fall to the bottom of the ocean. A scientist notices rock layers that have formed on the ocean bottom with fossils of some of these animals. What type of rock
1.Lava
2.igneous
3.sedimentary
4.metamorphic
Answers
Answer:
The answer is Sedimentary
Explanation:
Thank you so much if that helps you!!!!
What is the molar mass of silver oxide (Ag2O)
Answers
Answer:
231.735 g/mol
Explanation:
which statement is true regarding the conversion of arginine to ornithine in the urea cycle? a. the enzyme catalyzing this reaction is a hydrolase. b. this enzyme-catalyzed reaction occurs in the matrix. c. one of the products of this reaction is fumarate. d. the enzyme catalyzing this reaction is a ligase. e. the enzyme catalyzing this reaction is a hydrolase, and the reaction occurs in a matrix.
Answers
This reaction is catalyzed by an enzyme called a hydrolase, and it takes place in a matrix.
What enzyme does arginine's transformation into urea and ornithine?By converting l-arginine to l-ornithine and urea, the ureohydrolase arginase, an enzyme that contains manganese, catalyzes the last stage in the urea cycle to get rid of harmful ammonia.
Why is arginine necessary for the urea cycle?It is changed by the enzyme arginase into L-ornithine, a building block for polyamines and urea, both of which are necessary for the urea cycle. Arg is a precursor of creatine, which is responsible for Arg catabolism, the production of agmatine, and the synthesis of proteins. Creatine is crucial for the energy metabolism of muscle, neuron, and testicles.
To know more about enzyme catalyzing visit :-
https://brainly.com/question/11102534
#SPJ4
I need help ASAP!! Which agents of weathering and/or erosion are shown in the images?
Ex: water, ice, wind, gravity, growing plants, animals

Answers
1) Mechanical agent
2) Biological agent
3) Physical agents
4) Physical agents
What are the agents of weathering?
Weathering is the process by which rocks, minerals, and soils are broken down into smaller particles by physical, chemical, and biological agents.
These agents of weathering can act together or independently to break down rocks and minerals into smaller particles, which can then be transported by wind, water, and other geological processes to create new landforms and soil. Weathering plays an important role in the formation of soils and in the shaping of the Earth's surface over time.
Learn more about weathering:https://brainly.com/question/23449272
#SPJ1
Write the balanced symbol equation for the electrolysis of aluminium oxide to produce aluminium, and the reduction of iron oxide with carbon to produce iron.
Then use that to calculate the atom economy for each.
Answers
Answer:
Electrolysis of Al₂O₃: 4Al³⁺ (s) + 6O²⁺ (g) → 4Al (s) + 3O₂ (g)
Reduction of Elemental Fe: 2Fe₂O₃ (s) + 3C (s) → 4Fe (s) + 3CO₂ (g)
Atom Economy for Electrolysis of Al₂O₃: 52.9227%
Atom Economy for Reduction of Fe₂O₃: 62.8534%
Explanation:
Step 1: Define Compounds
Aluminum Oxide - Al₂O₃
Iron Oxide - Fe₂O₃
Step 2: RxN
Al₂O₃ (s) → Al (s) + O₂ (g)
Fe₂O₃ (s) + C (s) → Fe (s) + CO₂ (g)
Step 3: Balance RxN
2Al₂O₃ (s) → 4Al (s) + 3O₂ (g)
We need the same number of O on both sides (6 is the LCM)We will also need to balance the number of Al on both sides due to the change of O (4 reactant/product)This is ONLY the decomposition reaction for Aluminum oxide, NOT the electrolysis.
2Fe₂O₃ (s) + 3C (s) → 4Fe (s) + 3CO₂ (g)
We need the same number of O on both sides (6 is the LCM)We will also need to balance the number of Fe on both sides due to the change of O (4 reactant/product)We will also need to balance the number of C on both sides due to the change of O (3 reactant/product)This is the final single-replacement reaction for the reduction of Iron Oxide to Iron.
Step 4: Electrolysis of Al₂O₃
We will have to use oxidation-reduction reactions (half-reactions). Let's break up the reaction into it's elements.
Al³⁺ + ? → Al
To make the ion Al³⁺ turn into its neutral atom, we will need to add 3e⁻ to balance the half reactionAl³⁺ + 3e⁻ → Al
O²⁻ → O₂ + ?
Oxygen is a diatomic element, and in it's natural state is bonded to itself. We need to balance the half reaction2O²⁻ → O₂ + ?
We need to figure out how much electrons the ion O²⁻ loses to turn into its neutral atom. We see that we will need to lose 4e⁻2O²⁻ → O₂ + 4e⁻
Our half reactions:
Al³⁺ + 3e⁻ → Al
2O²⁻ → O₂ + 4e⁻
We now need balance the entire half reaction. Our LCM is 124 (Al³⁺ + 3e⁻ → Al) = 4Al³⁺ + 12e⁻ → 4Al
3 (2O²⁻ → O₂ + 4e⁻) = 6O²⁻ → 3O₂ + 12e⁻
Add the 2 half reactions4Al³⁺ + 12e⁻ + 6O²⁺ → 4Al + 3O₂ + 12e⁻
Cancel out spectator ions/e⁻ to get our final half reaction4Al³⁺ (s) + 6O²⁺ (g) → 4Al (s) + 3O₂ (g)
Step 5: Atom Economy
According to GCSE and my own deciphering, your equation for Atom Economy is essentially calculating for something similar to percent yield (but not quite).
\(Atom \hspace{3} Economy \hspace{3} = \hspace{3} \frac{Molar \hspace{3} Mass \hspace{3} of \hspace{3} Product}{Molar \hspace{3} Mass \hspace{3} of \hspace{3} All \hspace{3} Reactants} \cdot 100 \%\)
4Al³⁺ (s) + 6O²⁺ (g) → 4Al (s) + 3O₂ (g)Molar Mass of Al - 26.98 g/mol
Molar Mass of O - 16.00 g/mol
Reactants: 4(26.98 g/mol) + 6(16.00 g/mol) = 203.92 g/mol
Products (Al as end product): 4(26.98 g/mol) = 107.92 g/mol
\(Atom \hspace{3} Economy \hspace{3} = \hspace{3} \frac{107.92 \hspace{3} g/mol}{203.92 \hspace{3} g/mol} \cdot 100 \%=52.9227 \%\)
2Fe₂O₃ (s) + 3C (s) → 4Fe (s) + 3CO₂ (g)Molar Mass of Fe - 55.85 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of C - 12.01 g/mol
Reactants: 4(55.85 g/mol) + 6(16.00 g/mol) + 3(12.01 g/mol) = 355.43 g/mol
Products (Fe as end product): 4(55.85 g/mol) = 223.4 g/mol
\(Atom \hspace{3} Economy \hspace{3} = \hspace{3} \frac{223.4 \hspace{3} g/mol}{355.43 \hspace{3} g/mol} \cdot 100 \%=62.8534\%\)
Step 6: Check for significant figures
Since we are not given any values, we don't really need to change any numbers to fit sig fig rules.
If you could repeat the lab and make it better, what would you do differently and why?
There are always ways that labs can be improved. Now that you are a veteran of this lab and have experience with the procedure, offer some advice to the next scientist about what you suggest and why. Your answer should be at least two to three sentences in length.
need help with lab!- earth and space science 1
Answers
In order to obtain more accurate results as well as to improve the efficiency of the laboratory procedure, the following recommendations are given:
There should be accurate calibration of instrumentsThe samples should be properly labeledRepeated measurements should be takenHow can improvements be done to a lab to obtain better results?Improving laboratory results can be achieved through several strategies aimed at enhancing experimental conditions, equipment, procedures, and data analysis.
Some possible methods for improving laboratory performance:
Regularly calibrate and maintain laboratory instruments and equipment to ensure accuracy and reliability. Implement robust quality control measures by using appropriate standards, controls, and reference materials. Develop and follow standardized operating procedures for all experiments and tests.Proper labeling, preservation, and storage at appropriate temperatures.Learn more about lab procedures at: https://brainly.com/question/13517732
#SPJ1
which of the following is an example of a longitudinal wave

Answers
Answer:
Examples of longitudinal waves include: sound waves. ultrasound waves. seismic P-waves.
Explanation:
Task B: Use the following maps to complete the questions:
Plantsburgh
2090
30
Watertown
35920
988
Lake Ontario
Rochester
317
Buffalo
2911
20
29 960
18
Lake
Erie
-992-
Utica
42,
983)
39
Albany
Ithaca
-996
051
36
40 960
1000
38
1004
1008
1. Which type of pressure center is shown on the map?
a) Low pressure
b) High pressure
une used to collect this data
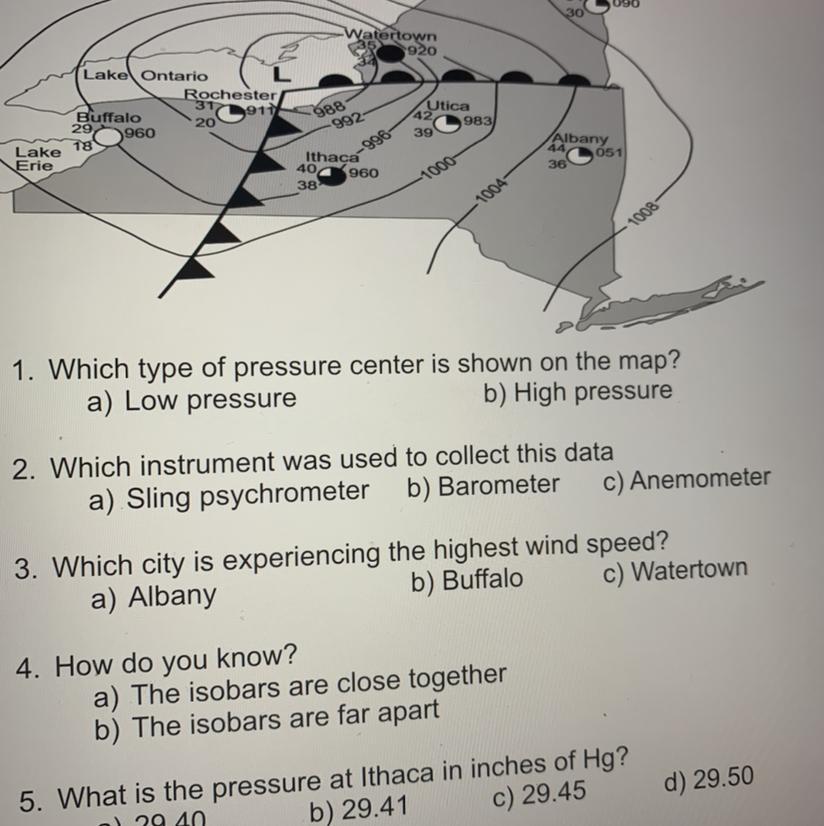
Answers
Answer:
1. B2. A3. B4. B5. CI HOPE IT HELPS :) 100% surenesscalculate the mass of oxalic acid(diprotic) crystals, h2c2o4.2h2o required to prepare 250.00 ml of a 0.200m acid solution.
Answers
The mass of oxalic acid dihydrate required to prepare 250.00 ml of a 0.200 M acid solution is 13.36 grams.
To calculate the mass of oxalic acid dihydrate required to prepare a 0.200 M solution, we need to first determine the molecular weight of the compound. The molecular weight of oxalic acid dihydrate is 126.07 g/mol. Next, we can use the formula for calculating the mass of a compound needed to prepare a solution:
mass = (molarity × volume × molecular weight) / 1000
Plugging in the values, we get:
mass = (0.200 mol/L × 0.250 L × 126.07 g/mol) / 1000 = 3.1535 g
However, we need to account for the fact that oxalic acid is diprotic, meaning each molecule has two acidic hydrogen atoms that can dissociate. Therefore, we need to multiply the result by 2:
mass = 3.1535 g × 2 = 6.307 g
Finally, since we are given the dihydrate form of oxalic acid, we need to add the mass of the two water molecules that are part of each molecule of the compound: mass = 6.307 g + 2 × 18.02 g/mol = 13.36 g
Therefore, the mass of oxalic acid dihydrate required to prepare 250.00 ml of a 0.200 M acid solution is 13.36 grams.
Learn more about oxalic acid here:
https://brainly.com/question/10967292
#SPJ11